
The genetic code is the set of rules used by living cells to translate information encoded within genetic material into proteins. Translation is accomplished by the ribosome, which links proteinogenic amino acids in an order specified by messenger RNA (mRNA), using transfer RNA (tRNA) molecules to carry amino acids and to read the mRNA three nucleotides at a time. The genetic code is highly similar among all organisms and can be expressed in a simple table with 64 entries.

Tryptophan is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG.
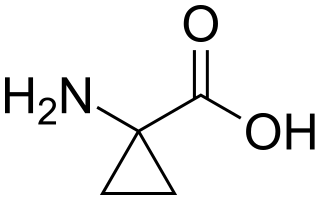
1-Aminocyclopropane-1-carboxylic acid (ACC) is a disubstituted cyclic α-amino acid in which a cyclopropane ring is fused to the Cα atom of the amino acid. It is a white solid. Many cyclopropane-substituted amino acids are known, but this one occurs naturally. Like glycine, but unlike most α-amino acids, ACC is not chiral.

Dehydroalanine is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As an amino acid residue, it is unusual because it has an unsaturated backbone.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis activity. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified, and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin. The tuberactinomycins target bacterial ribosomes, binding RNA and disrupting bacterial protein synthesis and certain forms of RNA splicing. Viomycin is produced by the actinomycete Streptomyces puniceus.

l-Kynurenine is a metabolite of the amino acid l-tryptophan used in the production of niacin.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.

Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis - the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) through a 2,3-bisphosphoglycerate intermediate. These enzymes are categorized into the two distinct classes of either cofactor-dependent (dPGM) or cofactor-independent (iPGM). The dPGM enzyme is composed of approximately 250 amino acids and is found in all vertebrates as well as in some invertebrates, fungi, and bacteria. The iPGM class is found in all plants and algae as well as in some invertebrate, fungi, and Gram-positive bacteria. This class of PGM enzyme shares the same superfamily as alkaline phosphatase.

Sialyltransferases are enzymes that transfer sialic acid to nascent oligosaccharide. Each sialyltransferase is specific for a particular sugar substrate. Sialyltransferases add sialic acid to the terminal portions of the sialylated glycolipids (gangliosides) or to the N- or O-linked sugar chains of glycoproteins.

Neutral and basic amino acid transport protein rBAT is a protein that in humans is encoded by the SLC3A1 gene.

Proton-coupled amino acid transporter 1 is a protein that in humans is encoded by the SLC36A1 gene.

Neutral amino acid transporter A is a protein that in humans is encoded by the SLC1A4 gene.

Y+L amino acid transporter 1 is a protein that in humans is encoded by the SLC7A7 gene.
Zwittermicin A is an antibiotic that has been identified from the bacterium Bacillus cereus UW85. It is a molecule of interest to agricultural industry because it has the potential to suppress plant disease due to its broad spectrum activity against certain gram positive and gram negative prokaryotic micro-organisms. The molecule is also of interest from a metabolic perspective because it represents a new structural class of antibiotic and suggests a crossover between polyketide and non-ribosomal peptide biosynthetic pathways. Zwittermicin A is linear aminopolyol.

ZM-241,385 is a high affinity antagonist ligand selective for the adenosine A2A receptor.

Proton-coupled amino acid transporter 2 is a protein which in humans is encoded by the SLC36A2 gene.
UDP-N-acetyl-2-amino-2-deoxyglucuronate dehydrogenase (EC 1.1.1.335, WlbA, WbpB) is an enzyme with systematic name UDP-N-acetyl-2-amino-2-deoxy-alpha-D-glucuronate:NAD+ 3-oxidoreductase. This enzyme catalyses the following chemical reaction:

N-Sulfinyl imines are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display useful stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen. The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.
Butyrolactol A is an organic chemical compound of interest for its potential use as an antifungal antibiotic.















