
A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.

Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. The reaction was reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912.
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .
Trichrome staining is a histological staining method that uses two or more acid dyes in conjunction with a polyacid. Staining differentiates tissues by tinting them in contrasting colours. It increases the contrast of microscopic features in cells and tissues, which makes them easier to see when viewed through a microscope.
Substances, mixtures and exposure circumstances in this list have been classified by the International Agency for Research on Cancer (IARC) as group 3: The agent is not classifiable as to its carcinogenicity to humans. This category is used most commonly for agents, mixtures and exposure circumstances for which the evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.

Dithiothreitol (DTT) is an organosulfur compound with the formula (CH CH2SH)2. A colorless compound, it is classified as a dithiol and a diol. DTT is redox reagent also known as Cleland's reagent, after W. Wallace Cleland. The reagent is commonly used in its racemic form. Its name derives from the four-carbon sugar, threose. DTT has an epimeric ('sister') compound, dithioerythritol (DTE).

Ponceau 2R, Xylidine ponceau, Ponceau G, Red R, Acid Red 26, Food Red 5, or C.I. 16150 is a red azo dye used in histology for staining. It is easily soluble in water and slightly in ethanol. It usually comes as a disodium salt.

Sodium bis(2-methoxyethoxy)aluminium hydride (SMEAH; trade names Red-Al, Synhydrid, Vitride) is a complex hydride reductant with the formula NaAlH2(OCH2CH2OCH3)2. The trade name Red-Al refers to its being a reducing aluminium compound. It is used predominantly as a reducing agent in organic synthesis. The compound features a tetrahedral aluminium center attached to two hydride and two alkoxide groups, the latter derived from 2-methoxyethanol. Commercial solutions are colorless/pale yellow and viscous. At low temperatures (below -60 °C), the solution solidifies to a glassy pulverizable substance with no sharp melting point.
Xylidine can refer to any of the six isomers of xylene amine, or any mixture of them.

3,5-Xylidine is the organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. It is used in the production of the dye Pigment Red 149.
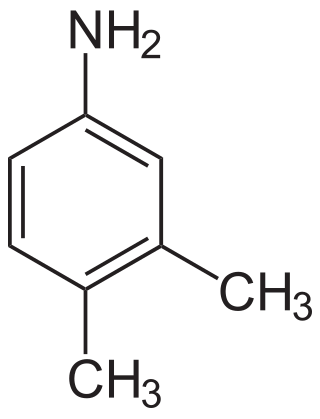
3,4-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless solid. It is a precursor for the production of riboflavin (vitamin B2).

2,6-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives are the anesthetics lidocaine, bupivacaine, mepivacaine, and etidocaine.
An antiknock agent is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs. The mixture known as gasoline or petrol, when used in high compression internal combustion engines, has a tendency to knock and/or to ignite early before the correctly timed spark occurs.
Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.
In chemistry, a crossover experiment is a method used to study the mechanism of a chemical reaction. In a crossover experiment, two similar but distinguishable reactants simultaneously undergo a reaction as part of the same reaction mixture. The products formed will either correspond directly to one of the two reactants or will include components of both reactants. The aim of a crossover experiment is to determine whether or not a reaction process involves a stage where the components of each reactant have an opportunity to exchange with each other.














