In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. Structurally, it is an ether with a methyl and phenyl group attached. Anisole is a standard reagent of both practical and pedagogical value.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.

Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.
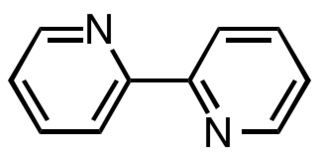
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.

Organoiridium chemistry is the chemistry of organometallic compounds containing an iridium-carbon chemical bond. Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid. They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
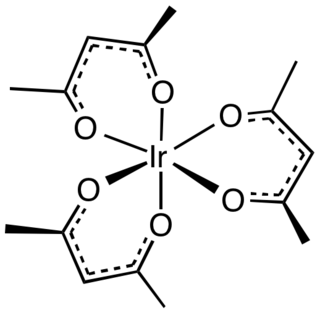
Iridium acetylacetonate is the iridium coordination complex with the formula Ir(O2C5H7)3, which is sometimes known as Ir(acac)3. The molecule has D3-symmetry. It is a yellow-orange solid that is soluble in organic solvents.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

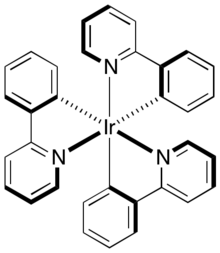
Tris(2-phenylpyridine)iridium, abbreviated [Ir(ppy)3] is the organoiridium complex with the formula Ir(C6H4-C5H4N)3. The complex, a yellow-green solid, is a derivative of Ir3+ bound to three monoanionic 2-pyridinylphenyl ligands. It is electroluminescent, emitting green light. The complex is observed with the facial stereochemistry, which is chiral.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.