In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction. Natural proteins as well as some common plastics such as nylon and PETE are formed in this way. Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers.

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.
The following outline is provided as an overview of and topical guide to organic chemistry:
Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.

Poly(vinyl alcohol) (PVOH, PVA, or PVAl) is a water-soluble synthetic polymer. It has the idealized formula [CH2CH(OH)]n. It is used in papermaking, textile warp sizing, as a thickener and emulsion stabilizer in polyvinyl acetate (PVAc) adhesive formulations, in a variety of coatings, and 3D printing. It is colourless (white) and odorless. It is commonly supplied as beads or as solutions in water. Without an externally added crosslinking agent, PVA solution can be gelled through repeated freezing-thawing, yielding highly strong, ultrapure, biocompatible hydrogels which have been used for a variety of applications such as vascular stents, cartilages, contact lenses, etc.

In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally-occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation.

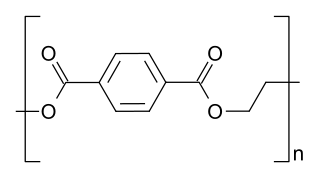
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Barrelene is a bicyclic organic compound with chemical formula C8H8 and systematic name bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel, with the staves being three ethylene units attached to two methine groups. It is the formal Diels–Alder adduct of benzene and acetylene. Due to its unusual molecular geometry, the compound is of considerable interest to theoretical chemists.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.

Polybutylene succinate (PBS) is a thermoplastic polymer resin of the polyester family. PBS is a biodegradable aliphatic polyester with properties that are comparable to polypropylene.
The Danheiser benzannulation is a chemical reaction used in organic chemistry to generate highly substituted phenols in a single step. It is named after Rick L. Danheiser who developed the reaction.
Expanding monomers are monomers which increase in volume (expand) during polymerization. They can be added to monomer formulations to counteract the usual volume shrinking to manufacture products with higher quality and durability. Volume Shrinkage is in first line for the unmeltable thermosets a problem, since those are of fixed shape after polymerization completed.
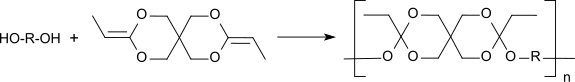
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.
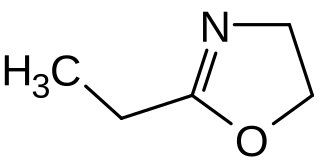
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.

2,2-Diethoxytetrahydrofuran is a cyclic orthoester which can be reacted with diols to biodegradable polyorthoesters.
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DVTOSU) is a bicyclic organic molecule having a central quaternary carbon atom with which two alicyclic rings are linked, each comprising five atoms. DVTOSU is a diallyl acetal and the precursor for the isomeric ketene acetal monomer 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane (DETOSU) which is a building block for polyorthoesters.

β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties (e.g. strength or degradation behaviour) to the (R)-poly-3-hydroxybutyrate originating from natural sources.
1,2,6-Hexanetriol is a trivalent alcohol with two primary and one secondary hydroxy group. It is similar to glycerol in many respects and is used as a substitute for glycerol in many applications due to its more advantageous properties, such as higher thermal stability and lower hygroscopicity.
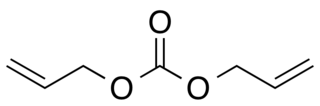
Diallyl carbonate (DAC) is a colorless liquid with a pungent odor. Its structure contains allyl groups and a functional carbonate group. The presence of double bonds in the allyl groups makes it reactive in various chemical processes. This compound plays a key role in the production of polymers, including polycarbonates and polyurethanes. Diallyl carbonate is soluble in ethanol, methanol, toluene, and chloroform. Diallyl carbonate reacts with amines, alcohols, and thiols.