The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base. The organoboron species is usually synthesized by hydroboration or carboboration, allowing for rapid generation of molecular complexity.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.
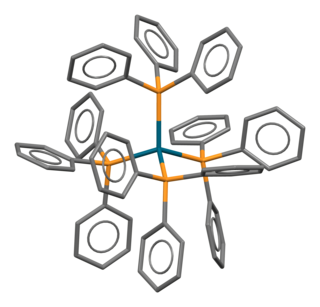
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound [Pd(P(C6H5)3)4], often abbreviated Pd(PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry.
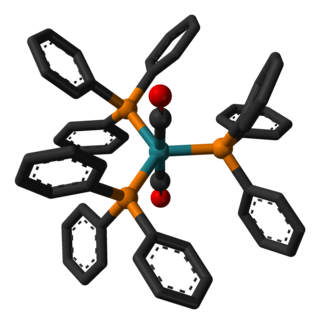
Dicarbonyltris(triphenylphosphine)ruthenium(0) or Roper's complex is a ruthenium metal carbonyl. In it, two carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium(0) center.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).
Martin Arthur Bennett FRS is an Australian inorganic chemist. He gained recognition for studies on the co-ordination chemistry of tertiary phosphines, olefins, and acetylenes, and the relationship of their behaviour to homogeneous catalysis.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.
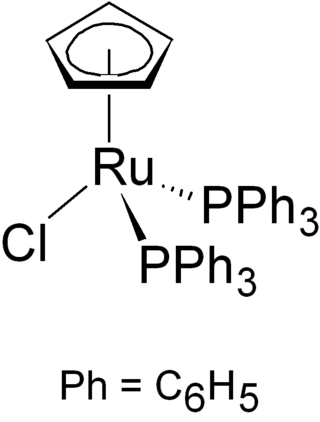
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.

Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

In chemistry, the hydrogenation of carbon–nitrogen double bonds is the addition of the elements of dihydrogen (H2) across a carbon–nitrogen double bond, forming amines or amine derivatives. Although a variety of general methods have been developed for the enantioselective hydrogenation of ketones, methods for the hydrogenation of carbon–nitrogen double bonds are less general. Hydrogenation of imines is complicated by both syn/anti isomerization and tautomerization to enamines, which may be hydrogenated with low enantioselectivity in the presence of a chiral catalyst. Additionally, the substituent attached to nitrogen affects both the reactivity and spatial properties of the imine, complicating the development of a general catalyst system for imine hydrogenation. Despite these challenges, methods have been developed that address particular substrate classes, such as N-aryl, N-alkyl, and endocyclic imines.
Gábor Laurenczy is a Hungarian-Swiss chemist and academic. He is a Professor Emeritus at the École Polytechnique Fédérale de Lausanne. He is academician, External Member of the Hungarian Academy of Sciences.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Dichlorobis(triphenylphosphine)nickel(II) refers to a pair of metal phosphine complexes with the formula NiCl2[P(C6H5)3]2. The compound exists as two isomers, a paramagnetic dark blue solid and a diamagnetic red solid. These complexes function as catalysts for organic synthesis.
In organic chemistry, the Murai reaction is an organic reaction that uses C-H activation to create a new C-C bond between a terminal or strained internal alkene and an aromatic compound using a ruthenium catalyst. The reaction, named after Shinji Murai, was first reported in 1993. While not the first example of C-H activation, the Murai reaction is notable for its high efficiency and scope. Previous examples of such hydroarylations required more forcing conditions and narrow scope.

















