
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG.

Threonine is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli. It is encoded by all the codons starting AC.

Carnitine is a quaternary ammonium compound involved in metabolism in most mammals, plants, and some bacteria. In support of energy metabolism, carnitine transports long-chain fatty acids into mitochondria to be oxidized for free energy production, and also participates in removing products of metabolism from cells. Given its key metabolic roles, carnitine is concentrated in tissues like skeletal and cardiac muscle that metabolize fatty acids as an energy source. Generally individuals, including strict vegetarians, synthesize enough L-carnitine in vivo.
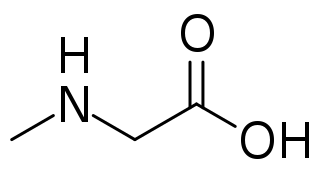
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life.
Fatty acid metabolism consists of various metabolic processes involving or closely related to fatty acids, a family of molecules classified within the lipid macronutrient category. These processes can mainly be divided into (1) catabolic processes that generate energy and (2) anabolic processes where they serve as building blocks for other compounds.
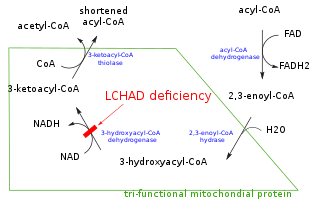
In biochemistry and metabolism, beta-oxidation is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA, which enters the citric acid cycle, and NADH and FADH2, which are co-enzymes used in the electron transport chain. It is named as such because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.

3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The anion is often termed as PGA when referring to the Calvin-Benson cycle. In the Calvin-Benson cycle, 3-phosphoglycerate is typically the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2 that is fixed. In glycolysis, 3-phosphoglycerate is an intermediate following the dephosphorylation (reduction) of 1,3-bisphosphoglycerate.
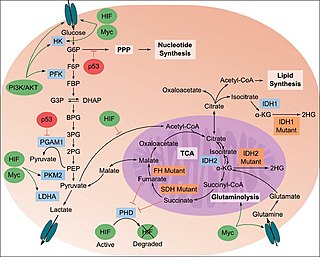
The study of the tumor metabolism, also known as tumor metabolome describes the different characteristic metabolic changes in tumor cells. The characteristic attributes of the tumor metabolome are high glycolytic enzyme activities, the expression of the pyruvate kinase isoenzyme type M2, increased channeling of glucose carbons into synthetic processes, such as nucleic acid, amino acid and phospholipid synthesis, a high rate of pyrimidine and purine de novo synthesis, a low ratio of Adenosine triphosphate and Guanosine triphosphate to Cytidine triphosphate and Uridine triphosphate, low Adenosine monophosphate levels, high glutaminolytic capacities, release of immunosuppressive substances and dependency on methionine.

β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid or BHB, is an organic compound and a beta hydroxy acid with the chemical formula CH3CH(OH)CH2CO2H; its conjugate base is β-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxybutyric acid is a chiral compound with two enantiomers: D-β-hydroxybutyric acid and L-β-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature. In humans, D-β-hydroxybutyric acid is one of two primary endogenous agonists of hydroxycarboxylic acid receptor 2 (HCA2), a Gi/o-coupled G protein-coupled receptor (GPCR).
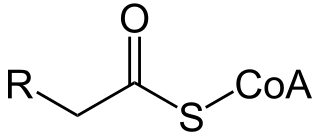
Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the universal biochemical energy carrier.
Fatty acid degradation is the process in which fatty acids are broken down into their metabolites, in the end generating acetyl-CoA, the entry molecule for the citric acid cycle, the main energy supply of living organisms, including bacteria and animals. It includes three major steps:

A broad classification for genetic disorders that result from an inability of the body to produce or utilize one enzyme that is required to oxidize fatty acids. The enzyme can be missing or improperly constructed, resulting in it not working. This leaves the body unable to produce energy within the liver and muscles from fatty acid sources.

Glycine betaine aldehyde, often simply called betaine aldehyde, is an intermediate in the metabolism of glycine, serine and threonine. The human aldehyde dehydrogenase stimulates the transformation of betaine aldehyde to glycine betaine. Betaine aldehyde is a substrate for choline dehydrogenase (mitochondrial).
Carnitine biosynthesis is a method for the endogenous production of L-carnitine, a molecule that is essential for energy metabolism. In humans and many other animals, L-carnitine is obtained from both diet and by biosynthesis. The carnitine biosynthesis pathway is highly conserved among many eukaryotes and some prokaryotes.
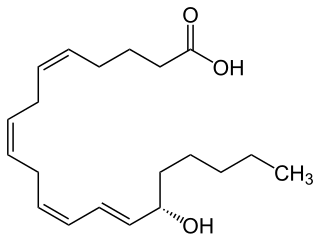
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5S,15S-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE, a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.

Methylene cyclopropyl acetic acid (MCPA) is found in lychee seeds and also a toxic metabolite in mammalian digestion after eating hypoglycin, present in the unripe ackee fruit, grown in Jamaica and in Africa. By blocking coenzyme A and carnitine, MPCA causes a decrease in β-oxidation of fatty acids, and hence gluconeogenesis.
Cytochrome P450 omega hydroxylases, also termed cytochrome P450 ω-hydroxylases, CYP450 omega hydroxylases, CYP450 ω-hydroxylases, CYP omega hydroxylase, CYP ω-hydroxylases, fatty acid omega hydroxylases, cytochrome P450 monooxygenases, and fatty acid monooxygenases, are a set of cytochrome P450-containing enzymes that catalyze the addition of a hydroxyl residue to a fatty acid substrate. The CYP omega hydroxylases are often referred to as monoxygenases; however, the monooxygenases are CYP450 enzymes that add a hydroxyl group to a wide range of xenobiotic and naturally occurring endobiotic substrates, most of which are not fatty acids. The CYP450 omega hydroxylases are accordingly better viewed as a subset of monooxygenases that have the ability to hydroxylate fatty acids. While once regarded as functioning mainly in the catabolism of dietary fatty acids, the omega oxygenases are now considered critical in the production or break-down of fatty acid-derived mediators which are made by cells and act within their cells of origin as autocrine signaling agents or on nearby cells as paracrine signaling agents to regulate various functions such as blood pressure control and inflammation.

β-Hydroxy β-methylbutyryl-coenzyme A (HMB-CoA), also known as 3-hydroxyisovaleryl-CoA, is a metabolite of L-leucine that is produced in the human body. Its immediate precursors are β-hydroxy β-methylbutyric acid (HMB) and β-methylcrotonoyl-CoA (MC-CoA). It can be metabolized into HMB, MC-CoA, and HMG-CoA in humans.















