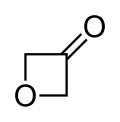
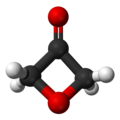
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes.
In chemical synthesis, click chemistry is a class of simple, atom-economy reactions commonly used for joining two molecular entities of choice. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, biomimetic and molecular machinery applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.

On-water reactions are a group of organic reactions that take place as an emulsion in water and have an unusual reaction rate acceleration compared with (i) the same reaction in an organic solvent, or (ii) the corresponding dry media reaction. This effect has been known for many years but in 2005 researchers in the group of K. Barry Sharpless published a systematic study into this phenomenon.
A dendralene is a discrete acyclic cross-conjugated polyene. The simplest dendralene is buta-1,3-diene (1) or [2]dendralene followed by [3]dendralene (2), [4]dendralene (3) and [5]dendralene (4) and so forth. [2]dendralene (butadiene) is the only one not cross-conjugated.

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.
In organic chemistry, a cycloalkyne is the cyclic analog of an alkyne. A cycloalkyne consists of a closed ring of carbon atoms containing one or more triple bonds. Cycloalkynes have a general formula CnH2n−4. Because of the linear nature of the C−C≡C−C alkyne unit, cycloalkynes can be highly strained and can only exist when the number of carbon atoms in the ring is great enough to provide the flexibility necessary to accommodate this geometry. Large alkyne-containing carbocycles may be virtually unstrained, while the smallest constituents of this class of molecules may experience so much strain that they have yet to be observed experimentally. Cyclooctyne is the smallest cycloalkyne capable of being isolated and stored as a stable compound. Despite this, smaller cycloalkynes can be produced and trapped through reactions with other organic molecules or through complexation to transition metals.

Maitotoxin is an extremely powerful biotoxin produced by Gambierdiscus toxicus, a dinoflagellate species. Maitotoxin has been shown to be more than one hundred thousand times more potent than VX nerve agent. Maitotoxin is so potent that it has been demonstrated that an intraperitoneal injection of 130 ng/kg was lethal in mice. Maitotoxin was named from the ciguateric fish Ctenochaetus striatus—called "maito" in Tahiti—from which maitotoxin was isolated for the first time. It was later shown that maitotoxin is actually produced by the dinoflagellate Gambierdiscus toxicus.

A circulene is a macrocyclic arene in which a central polygon is surrounded and fused by benzenoids. Nomenclature within this class of molecules is based on the number of benzene rings surrounding the core, which is equivalent to the size of the central polygon. Examples which have been synthesized include [5]circulene (corannulene), [6]circulene (coronene), [7]circulene, and [12]circulene (kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure. The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix rather than a closed ring.

Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests.

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance.

Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.

The A3 coupling (also known as A3 coupling reaction or the aldehyde-alkyne-amine reaction), coined by Prof. Chao-Jun Li of McGill University, is a type of multicomponent reaction involving an aldehyde, an alkyne and an amine which react to give a propargyl-amine.
Tellurium nitride describes chemical compounds of Te containing N3−. Efforts have been made toward the binary nitrides but the results are inconclusive and it appears that such materials are unstable. Still unconfirmed is Te4N4, which would be an analogue of tetraselenium tetranitride (Se4N4) and tetrasulfur tetranitride (S4N4). It has long been known that ammonia reacts with tellurium tetrachloride, which is similar to the method of synthesis of S4N4. The reaction of TeCl4 with a THF solution of N(SiMe3)3 gives a well-defined tellurium nitride [Te6N8(TeCl2)4(THF)4].
Organoneptunium chemistry is the chemical science exploring the properties, structure and reactivity of organoneptunium compounds, which are organometallic compounds containing a carbon to neptunium chemical bond. Several such compounds exist even though the element itself, neptunium, is man-made and highly radioactive: tricyclopentadienylneptunium-chloride, tetrakis(cyclopentadienyl)neptunium(IV) and neptunocene Np(C8H8)2.

Michal Hocek is a Czech chemist. He is a group leader at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences and a professor of organic chemistry at Charles University in Prague. He specializes in the chemistry and chemical biology of nucleosides, nucleotides, and nucleic acids.
Carbonyl olefin metathesis is a type of metathesis reaction that entails, formally, the redistribution of fragments of an alkene and a carbonyl by the scission and regeneration of carbon-carbon and carbon-oxygen double bonds respectively. It is a powerful method in organic synthesis using simple carbonyls and olefins and converting them into less accessible products with higher structural complexity.