 | |
| Names | |
|---|---|
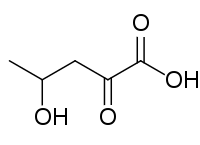
| Preferred IUPAC name 4-Hydroxy-2-oxopentanoic acid | |
| Other names 4-Hydroxy-2-ketopentanoic acid; 4-Hydroxy-2-ketovaleric acid; 4-Hydroxy-2-oxovaleric acid; 4-Hydroxy-2-oxopentanoate; 4-Hydroxy-2-ketopentanoate; 4-Hydroxy-2-ketovalerate; 4-Hydroxy-2-oxovalerate; HKP | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C5H8O4 | |
| Molar mass | 132.115 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
4-Hydroxy-2-oxopentanoaic acid, also known as 4-hydroxy-2-oxovalerate, is formed by the decarboxylation of 4-oxalocrotonate by 4-oxalocrotonate decarboxylase, is degraded by 4-hydroxy-2-oxovalerate aldolase, forming acetaldehyde and pyruvate and is reversibly dehydrated by 2-oxopent-4-enoate hydratase to 2-oxopent-4-enoate. [1]