GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
Ras, from "Rat sarcoma virus", is a family of related proteins that are expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. Ras is the prototypical member of the Ras superfamily of proteins, which are all related in three-dimensional structure and regulate diverse cell behaviours.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.

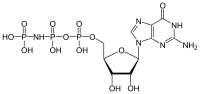
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.

Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine.

Cyclic guanosine monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP). cGMP acts as a second messenger much like cyclic AMP. Its most likely mechanism of action is activation of intracellular protein kinases in response to the binding of membrane-impermeable peptide hormones to the external cell surface. Through protein kinases activation, cGMP can relax smooth muscle. cGMP concentration in urine can be measured for kidney function and diabetes detection.

Guanylate cyclase is a lyase enzyme that converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and pyrophosphate:
The Rab family of proteins is a member of the Ras superfamily of small G proteins. Approximately 70 types of Rabs have now been identified in humans. Rab proteins generally possess a GTPase fold, which consists of a six-stranded beta sheet which is flanked by five alpha helices. Rab GTPases regulate many steps of membrane trafficking, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane fusion. These processes make up the route through which cell surface proteins are trafficked from the Golgi to the plasma membrane and are recycled. Surface protein recycling returns proteins to the surface whose function involves carrying another protein or substance inside the cell, such as the transferrin receptor, or serves as a means of regulating the number of a certain type of protein molecules on the surface.
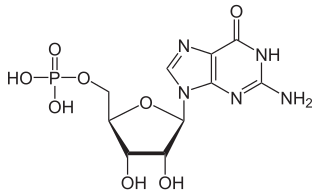
Guanosine monophosphate (GMP), also known as 5′-guanidylic acid or guanylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleoside monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation.

ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling.
In cell signalling, Son of Sevenless (SOS) refers to a set of genes encoding guanine nucleotide exchange factors that act on the Ras subfamily of small GTPases.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.
Signal-recognition-particle GTPase (EC 3.6.5.4) is an enzyme with systematic name GTP phosphohydrolase (protein-synthesis-assisting). This enzyme catalyses the following chemical reaction

Interferon-induced GTP-binding protein Mx1 is a protein that in humans is encoded by the MX1 gene.

RhoG is a small monomeric GTP-binding protein, and is an important component of many intracellular signalling pathways. It is a member of the Rac subfamily of the Rho family of small G proteins and is encoded by the gene RHOG.

RhoH is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RHOH.

Arf-GAP with GTPase, ANK repeat and PH domain-containing protein 3 is an enzyme that in humans is encoded by the AGAP3 gene.
EF-Ts is one of the prokaryotic elongation factors. It is found in human mitochondria as TSFM. It is similar to eukaryotic EF-1B.

In molecular biology, the guanylate-binding proteins family is a family of GTPases that is induced by interferon (IFN)-gamma. GTPases induced by IFN-gamma are key to the protective immunity against microbial and viral pathogens. These GTPases are classified into three groups: the small 47-KD immunity-related GTPases (IRGs), the Mx proteins, and the large 65- to 67-kd GTPases. Guanylate-binding proteins (GBP) fall into the last class.