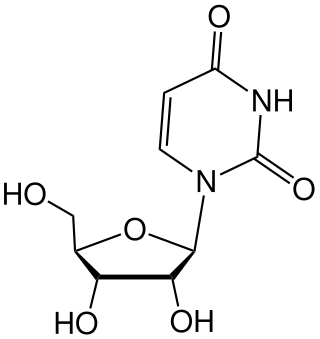
Uridine (symbol U or Urd) is a glycosylated pyrimidine analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1-glycosidic bond. The analog is one of the five standard nucleosides which make up nucleic acids, the others being adenosine, thymidine, cytidine and guanosine. The five nucleosides are commonly abbreviated to their symbols, U, A, dT, C, and G, respectively. However, thymidine is more commonly written as 'dT' ('d' represents 'deoxy') as it contains a 2'-deoxyribofuranose moiety rather than the ribofuranose ring found in uridine. This is because thymidine is found in deoxyribonucleic acid (DNA) and usually not in ribonucleic acid (RNA). Conversely, uridine is found in RNA and not DNA. The remaining three nucleosides may be found in both RNA and DNA. In RNA, they would be represented as A, C and G whereas in DNA they would be represented as dA, dC and dG.

Reticulocytes are immature red blood cells (RBCs). In the process of erythropoiesis, reticulocytes develop and mature in the bone marrow and then circulate for about a day in the blood stream before developing into mature red blood cells. Like mature red blood cells, in mammals, reticulocytes do not have a cell nucleus. They are called reticulocytes because of a reticular (mesh-like) network of ribosomal RNA that becomes visible under a microscope with certain stains such as new methylene blue and Romanowsky stain.
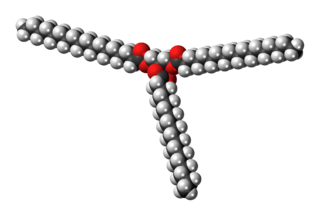
Stearin, or tristearin, or glyceryl tristearate is an odourless, white powder. It is a triglyceride derived from three units of stearic acid. Most triglycerides are derived from at least two and more commonly three different fatty acids. Like other triglycerides, stearin can crystallise in three polymorphs. For stearin, these melt at 54 (α-form), 65, and 72.5 °C (β-form).
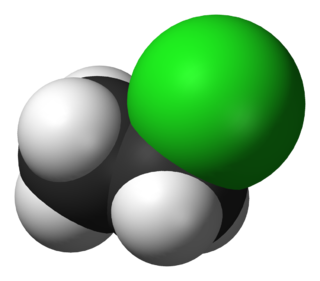
Chloroethane, commonly known as ethyl chloride, is a chemical compound with chemical formula CH3CH2Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Myristic acid is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nutmeg, from which it was first isolated in 1841 by Lyon Playfair.
This page provides supplementary chemical data on acetic acid.
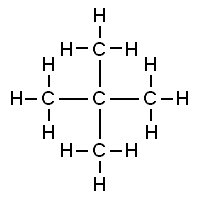
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.

Trimyristin is a saturated fat and the triglyceride of myristic acid with the chemical formula C45H86O6. Trimyristin is a white to yellowish-gray solid that is insoluble in water, but soluble in ethanol, acetone, benzene, chloroform, dichloromethane, ether, and TBME.

Zirconium nitride is an inorganic compound used in a variety of ways due to its properties.
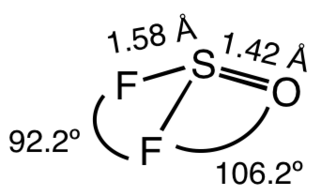
Thionyl fluoride is the inorganic compound with the formula SOF
2. This colourless gas is mainly of theoretical interest, but it is a product of the degradation of sulfur hexafluoride, an insulator in electrical equipment. The molecule adopts a distorted pyramidal structure, with Cs symmetry. The S-O and S-F distances are 1.42 and 1.58 Å, respectively. The O-S-F and F-S-F angles are 106.2 and 92.2°, respectively. Thionyl chloride and thionyl bromide have similar structures, although these compounds are liquid at room temperature. Mixed halides are also known, such as SOClF, thionyl chloride fluoride.

n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent

Zirconium carbide (ZrC) is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.

Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.

Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.
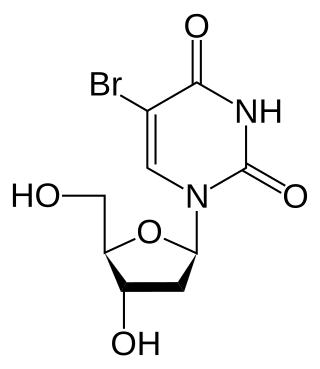
Bromodeoxyuridine is a synthetic nucleoside analogue with a chemical structure similar to thymidine. BrdU is commonly used to study cell proliferation in living tissues and has been studied as a radiosensitizer and diagnostic tool in people with cancer.
Decene is an organic compound with the chemical formula C10H20. Decene contains a chain of ten carbon atoms with one double bond, making it an alkene. There are many isomers of decene depending on the position and geometry of the double bond. Dec-1-ene is the only isomer of industrial importance. As an alpha olefin, it is used as a comonomer in copolymers and is an intermediate in the production of epoxides, amines, oxo alcohols, synthetic lubricants, synthetic fatty acids and alkylated aromatics.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.

Tripalmitin is a triglyceride derived from the fatty acid palmitic acid.
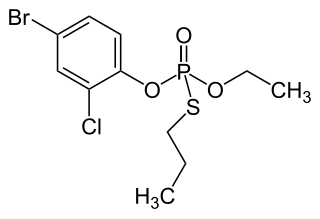
Profenofos is an organophosphate insecticide. It is a liquid with a pale yellow to amber color and a garlic-like odor. It was first registered in the United States in 1982. As of 2015, it was not approved in the European Union.















