
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
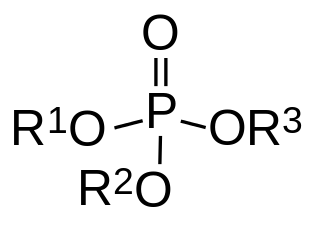
In organic chemistry, organophosphates are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
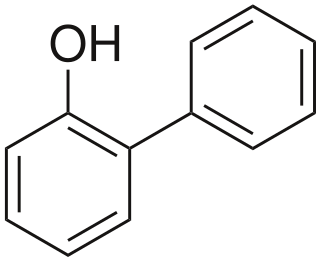
2-Phenylphenol, or o-phenylphenol, is an organic compound. In terms of structure, it is one of the monohydroxylated isomers of biphenyl. It is a white solid. It is a biocide used as a preservative with E number E231 and under the trade names Dowicide, Torsite, Fungal, Preventol, Nipacide and many others.

Oxazines are heterocyclic organic compounds containing one oxygen and one nitrogen atom in a cyclohexa-1,4-diene ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds.
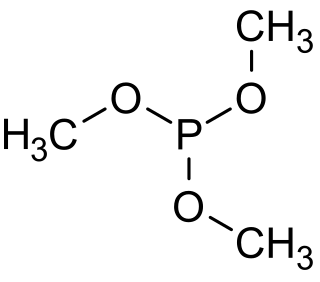
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into molecules, which includes many ligands. Like other halophosphines, Ph2PCl is reactive with many nucleophiles such as water and easily oxidized even by air.

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.

Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is an organophosphorus compound with the chemical formula [P(CH2OH)4]Cl. It is a white water-soluble salt with applications as a precursor to fire-retardant materials and as a microbiocide in commercial and industrial water systems.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
Fire-safe polymers are polymers that are resistant to degradation at high temperatures. There is need for fire-resistant polymers in the construction of small, enclosed spaces such as skyscrapers, boats, and airplane cabins. In these tight spaces, ability to escape in the event of a fire is compromised, increasing fire risk. In fact, some studies report that about 20% of victims of airplane crashes are killed not by the crash itself but by ensuing fires. Fire-safe polymers also find application as adhesives in aerospace materials, insulation for electronics, and in military materials such as canvas tenting.
Kaushal Kishore (1942–1999) was an Indian polymer chemist and head of the department of inorganic and physical Chemistry at the Indian Institute of Science (IISc). He was known for his researches on thermochemistry and combustion of polymers. and was an elected fellow of the National Academy of Sciences, India, Indian National Science Academy, and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1988, for his contributions to chemical sciences.

Stibinin, also known as stibabenzene, is an organic chemical compound. Stibinin has the chemical formula C5H5Sb. The molecule, stibinin, is a derivative of benzene, with one of the carbon atoms in the 6-membered ring replaced by an antimony (Sb) atom. Stibinin is a molecule that is considered to be an organoantimony compound due to it containing carbon, hydrogen, and antimony atoms.















