
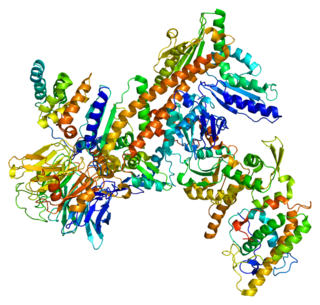
Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport.

Actin beta is one of six different actin isoforms which have been identified in humans. This is one of the two nonmuscle cytoskeletal actins. Actins are highly conserved proteins that are involved in cell motility, structure and integrity. Alpha actins are a major constituent of the contractile apparatus.

Dynactin subunit 1 is a protein that in humans is encoded by the DCTN1 gene.
(See also: List of proteins in the human body)

Tubulin beta-2A chain is a protein that in humans is encoded by the TUBB2A gene.

Dynactin is a 23 subunit protein complex that acts as a co-factor for the microtubule motor cytoplasmic dynein-1. It is built around a short filament of actin related protein-1 (Arp1).
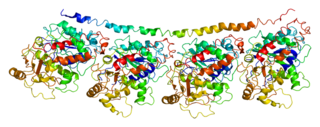
Tubulin alpha-4A chain is a protein that in humans is encoded by the TUBA4A gene.

Spectrin beta chain, brain 1 is a protein that in humans is encoded by the SPTBN1 gene.

Dynactin subunit 2 is a protein that in humans is encoded by the DCTN2 gene

Dynein light chain Tctex-type 1 is a protein that in humans is encoded by the DYNLT1 gene.

Bicaudal D cargo adaptor 2 is a protein that in humans is encoded by the BICD2 gene.

Spectrin beta chain, brain 2 is a protein that in humans is encoded by the SPTBN2 gene.

Actin-related protein 2/3 complex subunit 1A is a protein that in humans is encoded by the ARPC1A gene.

Dynactin subunit 3 is a protein that in humans is encoded by the DCTN3 gene.

Beta-centractin is a protein that in humans is encoded by the ACTR1B gene.

Arp2/3 complex is a seven-subunit protein complex that plays a major role in the regulation of the actin cytoskeleton. It is a major component of the actin cytoskeleton and is found in most actin cytoskeleton-containing eukaryotic cells. Two of its subunits, the Actin-Related Proteins ARP2 and ARP3, closely resemble the structure of monomeric actin and serve as nucleation sites for new actin filaments. The complex binds to the sides of existing ("mother") filaments and initiates growth of a new ("daughter") filament at a distinctive 70 degree angle from the mother. Branched actin networks are created as a result of this nucleation of new filaments. The regulation of rearrangements of the actin cytoskeleton is important for processes like cell locomotion, phagocytosis, and intracellular motility of lipid vesicles.
In molecular biology, DCTN6 is that subunit of the dynactin protein complex that is encoded by the p27 gene. Dynactin is the essential component for microtubule-based cytoplasmic dynein motor activity in intracellular transport of a variety of cargoes and organelles.

Dynactin subunit 4 is a protein that in humans is encoded by the DCTN4 gene.

Intracellular transport is the movement of vesicles and substances within a cell. Intracellular transport is required for maintaining homeostasis within the cell by responding to physiological signals. Proteins synthesized in the cytosol are distributed to their respective organelles, according to their specific amino acid’s sorting sequence. Eukaryotic cells transport packets of components to particular intracellular locations by attaching them to molecular motors that haul them along microtubules and actin filaments. Since intracellular transport heavily relies on microtubules for movement, the components of the cytoskeleton play a vital role in trafficking vesicles between organelles and the plasma membrane by providing mechanical support. Through this pathway, it is possible to facilitate the movement of essential molecules such as membrane‐bounded vesicles and organelles, mRNA, and chromosomes.

Erika L F. Holzbaur is an American biologist who is the William Maul Measey Professor of Physiology at University of Pennsylvania Perelman School of Medicine. Her research considers the dynamics of organelle motility along cytoskeleton of cells. She is particularly interested in the molecular mechanisms that underpin neurodegenerative diseases.
























