
Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

9,10-Diphenylanthracene is a polycyclic aromatic hydrocarbon. It has the appearance of a slightly yellow powder. 9,10-Diphenylanthracene is used as a sensitiser in chemiluminescence. In lightsticks it is used to produce blue light. It is a molecular organic semiconductor, used in blue OLEDs and OLED-based displays.

Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents. It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.

In the field of organic chemistry, a polycyclic compound is an organic compound featuring several closed rings of atoms, primarily carbon. These ring substructures include cycloalkanes, aromatics, and other ring types. They come in sizes of three atoms and upward, and in combinations of linkages that include tethering, fusing, links via a single atom, bridged compounds, and longifolene. Though poly- literally means "many", there is some latitude in determining how many rings are required to be considered polycyclic; many smaller rings are described by specific prefixes, and so while it can refer to these, the title term is used with most specificity when these alternative names and prefixes are unavailable.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5–6 mg/kg.
Mycobacterium vanbaalenii is a rapidly growing mycobacterium that can use polycyclic aromatic hydrocarbons. It was first isolated from petroleum-contaminated estuarine sediments and has been shown by 16S rRNA gene sequencing to be closely related to Mycobacterium aurum and Mycobacterium vaccae. M. vanbaalenii has potential use in the bioremediation of polycyclic aromatic hydrocarbon contaminated environmental sites. Etymology: vanbaalenii of Van Baalen, in memory of Dr Chase Van Baalen, late Professor at The University of Texas Marine Science Institute, Port Aransas Marine Laboratory, Port Aransas, TX, USA.
In organic and physical organic chemistry, Clar's rule is an empirical rule that relates the chemical stability of a molecule with its aromaticity. It was introduced in 1972 by the Austrian organic chemist Erich Clar in his book The Aromatic Sextet. The rule states that given a polycyclic aromatic hydrocarbon, the resonance structure most important to characterize its properties is that with the largest number of aromatic π-sextets i.e. benzene-like moieties.

1H-Phenalene, often called simply phenalene is a polycyclic aromatic hydrocarbon (PAH). Like many PAHs, it is an atmospheric pollutant formed during the combustion of fossil fuels. It is the parent compound for the phosphorus-containing phosphaphenalenes.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

Benz[a]anthracene or benzo[a]anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is produced during incomplete combustion of organic matter.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), bendo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.
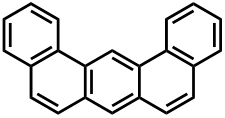
Dibenz[a,j]anthracene or Benzo[m]tetraphene or 1,2:7,8-Dibenzanthracene is an organic compound with the chemical formula C22H14. It belongs to the class of polycyclic aromatic hydrocarbons (PAHs) and is formed whenever there is incomplete combustion of organic matter. The IARC (International Agency for Research on Cancer) has classified it as possibly carcinogenic to humans, grouped into IARC group 2B.
Gallaecimonas is a recently described genus of bacteria. The first described species of this genus was Gallaecimonas pentaromativorans gen. nov., sp. nov. isolated by Rodríguez Blanco et al. in 2010 from intertidal sediments of the ria of Corcubión. It is a Gram-negative, rod-shaped, halotolerant bacterium in the class Gammaproteobacteria. It can degrade high molecular mass polycyclic aromatic hydrocarbons of 4 and 5 rings. The 16S rRNA gene sequences of the type strain CEE_131(T) proved to be distantly related to those of Rheinheimera and Serratia. Its G+C content was 41.7 mol%.

Benz[e]acephenanthrylene is an organic compound with the chemical formula C20H12. It is a polycyclic aromatic hydrocarbon (PAH) made of four benzene rings around a 5-membered ring.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
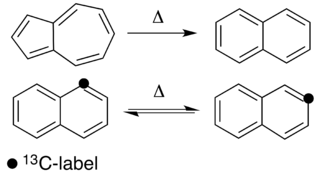
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.

Bicalicene is polycyclic hydrocarbon with chemical formula C16H8, composed of two cyclopentadiene and two cyclopropene rings linked into a larger eight-membered ring. There are two isomers: cis-bicalicene and trans-bicalicene. It is a dimer of calicene.

Staci Simonich is an American environmental scientist who is a professor and dean for the College of Agricultural Sciences at Oregon State University. Her research considers how chemicals move through the environment. She was appointed Fellow of the American Association for the Advancement of Science in 2021.

Indeno[1,2,3-cd]pyrene is a polycyclic aromatic hydrocarbon (PAH), one of 16 PAHs generally measured in studies of environmental exposure and air pollution. Many compounds of this class are formed when burning coal, oil, gas, wood, household waste and tobacco, and can bind to or form small particles in the air. The compounds are known to have toxic, mutagenic and/or carcinogenic properties. Over 100 different PAHs have been identified in environmental samples. One of these 16 is Indeno[1,2,3-cd]pyrene (IP). IP is the combination of an indeno molecule and a pyrene molecule with a fluoranthene network. In 1962, the National Cancer Institute reported that indeno[1,2,3-cd]pyrene has a slight tumor activity. This was confirmed in 1973 by the IARC in mice testing.

















