| |
| Names | |
|---|---|
| Preferred IUPAC name 1-(4-Methoxyphenyl)ethan-1-one | |
| Other names 4-Acetylanisole; para-Acetanisole; 4-Methoxyacetophenone; Linarodin; Novatone; Vananote; Castoreum anisole; 4-Methoxyphenyl methyl ketone | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.002.560 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.177 g·mol−1 |
| Appearance | White solid [1] |
| Density | 1.094 g/cm3 |
| Melting point | 38.2 °C (100.8 °F; 311.3 K) [2] |
| Boiling point | 254 °C (489 °F; 527 K) [2] |
| 2470 mg/L [3] | |
| Hazards | |
| Flash point | 138 °C (280 °F) [4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
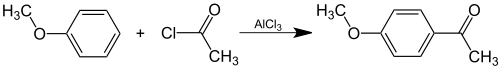
Acetanisole is an organic compound with the formula CH3OC6H4C(O)CH3. It can be viewed as derivative of acetophenone and of anisole. It has an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition the odor of acetanisole is sometimes described as butter-like or caramel-like. [4] It is used commercially in some soap fragrances. It is a component of anise oil. [1]