 | |
 | |
| Names | |
|---|---|
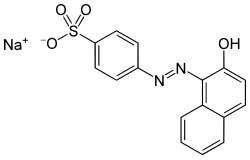
| IUPAC name sodium 4-[(2E)-2-(2-oxonaphthalen-1-ylidene)hydrazinyl]benzenesulfonate | |
| Other names 2-naphthol orange, Orange II, CI 15510, D&C Orange 4, COLIPA C015 | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.010.182 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C16H11N2NaO4S (sodium salt) | |
| Molar mass | 350.32 g/mol |
| Density | 1.525 g/cm3 |
| Melting point | 164 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Acid Orange 7, also known as 2-naphthol orange is an azo dye. It is used for dyeing wool.
