Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer.
L-selectin, also known as CD62L, is a cell adhesion molecule found on the cell surface of leukocytes, and the blastocyst. It is coded for in the human by the SELL gene. L-selectin belongs to the selectin family of proteins, which recognize sialylated carbohydrate groups containing a Sialyl LewisX (sLeX) determinant. L-selectin plays an important role in both the innate and adaptive immune responses by facilitating leukocyte-endothelial cell adhesion events. These tethering interactions are essential for the trafficking of monocytes and neutrophils into inflamed tissue as well as the homing of lymphocytes to secondary lymphoid organs. L-selectin is also expressed by lymphoid primed hematopoietic stem cells and may participate in the migration of these stem cells to the primary lymphoid organs. In addition to its function in the immune response, L-selectin is expressed on embryonic cells and facilitates the attachment of the blastocyst to the endometrial endothelium during human embryo implantation.

Vascular cell adhesion protein 1 also known as vascular cell adhesion molecule 1 (VCAM-1) or cluster of differentiation 106 (CD106) is a protein that in humans is encoded by the VCAM1 gene. VCAM-1 functions as a cell adhesion molecule.
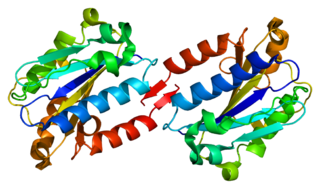
Integrin, alpha L , also known as ITGAL, is a protein that in humans is encoded by the ITGAL gene. CD11a functions in the immune system. It is involved in cellular adhesion and costimulatory signaling. It is the target of the drug efalizumab.
High endothelial venules (HEV) are specialized post-capillary venules characterized by plump endothelial cells as opposed to the usual flatter endothelial cells found in regular venules. HEVs enable lymphocytes circulating in the blood to directly enter a lymph node.

Chemokine ligand 21 (CCL21) is a small cytokine belonging to the CC chemokine family. This chemokine is also known as 6Ckine, exodus-2, and secondary lymphoid-tissue chemokine (SLC). CCL21 elicits its effects by binding to a cell surface chemokine receptor known as CCR7. The main function of CCL21 is to guide CCR7 expressing leukocytes to the secondary lymphoid organs, such as lymph nodes and Peyer´s patches.
Lymphocyte homing receptors are cell adhesion molecules expressed on lymphocyte cell membranes that recognize addressins on target tissues. Lymphocyte homing refers to adhesion of the circulating lymphocytes in blood to specialized endothelial cells within lymphoid organs. These diverse tissue-specific adhesion molecules on lymphocytes and on endothelial cells contribute to the development of specialized immune responses.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Integrin, alpha E (ITGAE) also known as CD103 is an integrin protein that in human is encoded by the ITGAE gene. CD103 binds integrin beta 7 to form the complete heterodimeric integrin molecule αEβ7, which has no distinct name. The αEβ7 complex is often referred to as "CD103" though this strictly refers only to the αE chain. Note that the β7 subunit can bind with other integrin α chains, such as α4 (CD49d).

C-C chemokine receptor type 7 is a protein that in humans is encoded by the CCR7 gene. Two ligands have been identified for this receptor: the chemokines ligand 19 (CCL19/ELC) and ligand 21 (CCL21). The ligands have similar affinity for the receptor, though CCL19 has been shown to induce internalisation of CCR7 and desensitisation of the cell to CCL19/CCL21 signals. CCR7 is a transmembrane protein with 7 transmembrane domains, which is coupled with heterotrimeric G proteins, which transduce the signal downstream through various signalling cascades. The main function of the receptor is to guide immune cells to immune organs by detecting specific chemokines, which these tissues secrete.

Intercellular adhesion molecule 3 (ICAM3) also known as CD50, is a protein that in humans is encoded by the ICAM3 gene. The protein is constitutively expressed on the surface of leukocytes, which are also called white blood cells and are part of the immune system. ICAM3 mediates adhesion between cells by binding to specific integrin receptors. It plays an important role in the immune cell response through its facilitation of interactions between T cells and dendritic cells, which allows for T cell activation. ICAM3 also mediates the clearance of cells undergoing apoptosis by attracting and binding macrophages, a type of cell that breaks down infected or dying cells through a process known as phagocytosis, to apoptotic cells.

Intercellular adhesion molecule 2 (ICAM2), also known as CD102, is a human gene, and the protein resulting from it.

C-type lectin domain family 4 member M is a protein that in humans is encoded by the CLEC4M gene. CLEC4M has also been designated as CD299.

Integrin beta-7 is an integrin protein that in humans is encoded by the ITGB7 gene. It can pair with ITGA4 (CD49d) to form the heterodimeric integrin receptor α4β7, or with ITGAE (CD103) to form αEβ7.
The following outline is provided as an overview of and topical guide to immunology:
IgSF CAMs are cell adhesion molecules that belong to Immunoglobulin superfamily. It is regarded as the most diverse superfamily of CAMs. This family is characterized by their extracellular domains containing Ig-like domains. The Ig domains are then followed by Fibronectin type III domain repeats and IgSFs are anchored to the membrane by a GPI moiety. This family is involved in both homophilic or heterophilic binding and has the ability to bind integrins or different IgSF CAMs.

In molecular biology, the adhesin molecule (immunoglobulin-like) is a protein domain. This domain is found in mucosal vascular addressin cell adhesion molecule 1 proteins (MAdCAM-1). These are cell adhesion molecules expressed on the endothelium in mucosa that guide the specific homing of lymphocytes into mucosal tissues. MAdCAM-1 belongs to a subclass of the immunoglobulin superfamily (IgSF), the members of which are ligands for integrins. The crystal structure of this domain has been reported; it adopts an immunoglobulin-like beta-sandwich structure, with seven strands arranged in two beta-sheets in a Greek-key topology.
Lymph node stromal cells are essential to the structure and function of the lymph node whose functions include: creating an internal tissue scaffold for the support of hematopoietic cells; the release of small molecule chemical messengers that facilitate interactions between hematopoietic cells; the facilitation of the migration of hematopoietic cells; the presentation of antigens to immune cells at the initiation of the adaptive immune system; and the homeostasis of lymphocyte numbers. Stromal cells originate from multipotent mesenchymal stem cells.
Gut-specific homing is the mechanism by which activated T cells and antibody-secreting cells (ASCs) are targeted to both inflamed and non-inflamed regions of the gut in order to provide an effective immune response. This process relies on the key interaction between the integrin α4β7 and the addressin MadCAM-1 on the surfaces of the appropriate cells. Additionally, this interaction is strengthened by the presence of CCR9, a chemokine receptor, which interacts with TECK. Vitamin A-derived retinoic acid regulates the expression of these cell surface proteins.
Integrin α4β7 is an integrin heterodimer composed of CD49d (alpha-4) subunit and beta-7 subunit noncovalently linked. LPAM-1 is expressed on the cell surface of leukocytes. This receptor is involved in lymphocyte trafficking pathway to site of inflammation in intestinal tissues.