Related Research Articles

Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
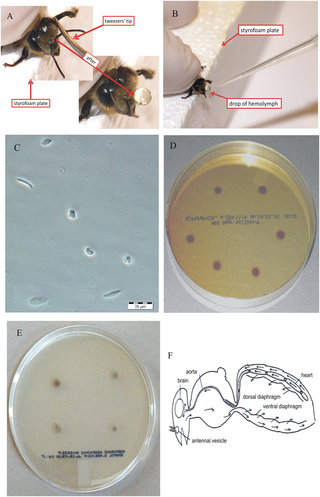
Hemolymph, or haemolymph, is a fluid, analogous to the blood in vertebrates, that circulates in the interior of the arthropod (invertebrate) body, remaining in direct contact with the animal's tissues. It is composed of a fluid plasma in which hemolymph cells called hemocytes are suspended. In addition to hemocytes, the plasma also contains many chemicals. It is the major tissue type of the open circulatory system characteristic of arthropods. In addition, some non-arthropods such as mollusks possess a hemolymphatic circulatory system.

Cholecystokinin is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of digestive enzymes and bile from the pancreas and gallbladder, respectively.
Neuropeptides are chemical messengers made up of small chains of amino acids that are synthesized and released by neurons. Neuropeptides typically bind to G protein-coupled receptors (GPCRs) to modulate neural activity and other tissues like the gut, muscles, and heart.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Poneratoxin is a paralyzing neurotoxic peptide made by the bullet ant Paraponera clavata. It prevents inactivation of voltage gated sodium channels and therefore blocks synaptic transmission in the central nervous system. Specifically, poneratoxin acts on voltage gated sodium channels in skeletal muscle fibers, causing paralysis, and nociceptive fibers, causing pain. It is rated as a 4 plus on the Schmidt sting pain index, the highest possible rating with that system, and its effects can cause waves of pain up to twelve hours after a single sting. It is additionally being studied for its uses in biological insecticides.
A polyproline helix is a type of protein secondary structure which occurs in proteins comprising repeating proline residues. A left-handed polyproline II helix is formed when sequential residues all adopt (φ,ψ) backbone dihedral angles of roughly and have trans isomers of their peptide bonds. This PPII conformation is also common in proteins and polypeptides with other amino acids apart from proline. Similarly, a more compact right-handed polyproline I helix is formed when sequential residues all adopt (φ,ψ) backbone dihedral angles of roughly and have cis isomers of their peptide bonds. Of the twenty common naturally occurring amino acids, only proline is likely to adopt the cis isomer of the peptide bond, specifically the X-Pro peptide bond; steric and electronic factors heavily favor the trans isomer in most other peptide bonds. However, peptide bonds that replace proline with another N-substituted amino acid are also likely to adopt the cis isomer.
Physalaemin is a tachykinin peptide obtained from the Physalaemus frog, closely related to substance P. Its structure was first elucidated in 1964.

Galanin is a neuropeptide encoded by the GAL gene, that is widely expressed in the brain, spinal cord, and gut of humans as well as other mammals. Galanin signaling occurs through three G protein-coupled receptors.

Blood sugar regulation is the process by which the levels of blood sugar, the common name for glucose dissolved in blood plasma, are maintained by the body within a narrow range.

Proctolin is a neuropeptide present in insects and crustaceans. It was first found in Periplaneta americana, a species of cockroach in 1975. Proctolin was extracted from 125,000 cockroaches and the Edman degradation was carried out on the sample to determine the amino acid sequence, which is Arg-Tyr-Leu-Pro-Thr.
Chemosensory proteins (CSPs) are small soluble proteins which mediate olfactory recognition at the periphery of sensory receptors in insects, similarly to odorant-binding proteins. The typical structure of CSPs is made of six or seven α-helical chains of about 110-120 amino acids, including four cysteines that build two small loops, two adjacent disulfide bridges, and a globular "prism-like" functional structure [5]. Three CSP structures have been solved in moths and locusts [5-8].

Crustacean cardioactive peptide (CCAP) is a highly conserved, amidated cyclic nonapeptide with the primary structure PFCNAFTGC-NH2 (ProPheCysAsnAlaPheThrGlyCys-NH2) and a disulfide bridge between Cys3 and Cys9. It is found in crustaceans and insects where it behaves as a cardioaccelerator, neuropeptide transmitter for other areas of the nervous system and a hormone. CCAP was first isolated from the pericardial organs of the shore crab Carcinus maenas, where it has a role in regulating heartbeat. It was assumed that this was the peptide's main function and its name reflects this.
Corazonin is a highly conserved neuropeptide found in many insects, in particular locusts and cockroaches.
Bursicon is an insect hormone which mediates tanning in the cuticle of adult flies. This hormone was identified by Gottfried S. Fraenkel with his student Catherine Hsiao in 1965.
RVD-Hpα (pepcan-12) is an endogenous neuropeptide found in human and mammalian brain, which was originally proposed to act as a selective agonist for the CB1 cannabinoid receptor. It is a 12-amino acid polypeptide having the amino acid sequence Arg-Val-Asp-Pro-Val-Asn-Phe-Lys-Leu-Leu-Ser-His and is an N-terminal extended form of hemopressin, a 9-AA polypeptide derived from the α1 subunit of hemoglobin which has previously been shown to act as a CB1 inverse agonist. All three polypeptides have been isolated from various mammalian species, with RVD-Hpα being one of the more abundant neuropeptides expressed in mouse brain, and these neuropeptides represent a new avenue for cannabinoid research distinct from the previously known endogenous lipid-derived cannabinoid agonists such as anandamide. Recently it was shown that RVD-Hpα (also called Pepcan-12) is a potent negative allosteric modulator at CB1 receptors, together with other newly described N-terminally extended peptides (pepcans).

α-Endorphin (alpha-endorphin) is an endogenous opioid peptide with a length of 16 amino acids, and the amino acid sequence: Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr. With the use of mass spectrometry, Nicholas Ling was able to determine the primary sequence of a-endorphin.
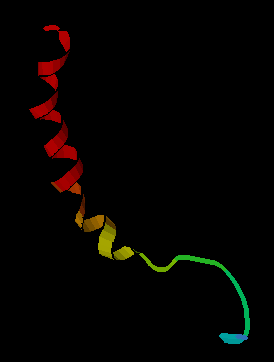
In molecular biology, the crustacean neurohormone family of proteins is a family of neuropeptides expressed by arthropods. The family includes the following types of neurohormones:

Pacifastin is a family of serine proteinase inhibitors found in arthropods. Pacifastin inhibits the serine peptidases trypsin and chymotrypsin.
Lipophorin is a lipid-carrying protein of insects, first identified in 1981, and is the major lipoprotein in the plasma of insects. Lipophorin has been identified in all insect species, in every life stage. Recently, additional nomenclature has been introduced to designate specific lipophorin subspecies that differ in lipid and/or apoprotein content. The concentration of lipophorin subspecies changes can be considered to reflect the physiological state of the organism with respect to lipid metabolism. The versatility of this particle concerning its lipid binding capacity may be unparalleled in nature.
References
- 1 2 3 "Chapter Eleven - HEMOLYMPH TRANSPORT OF METABOLITES: ENDOCRINE REGULATION". June 7, 2008. Archived from the original on 2008-06-07.
- 1 2 Stone, Judith V.; Mordue, William; Batley, Karen E.; Morris, Howard R. (September 1976). "Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight". Nature. 263 (5574): 207–211. Bibcode:1976Natur.263..207S. doi:10.1038/263207a0. ISSN 0028-0836. PMID 958472. S2CID 4226013.
- ↑ König, Simone (30 July 2005). "Prediction of insect adipokinetic hormone sequences assists in de novo structure elucidation". Rapid Communications in Mass Spectrometry. 19 (14): 2103–2104. Bibcode:2005RCMS...19.2103K. doi:10.1002/rcm.2017. ISSN 0951-4198. PMID 15988719.
- ↑ Eisenacher, Martin; de Braaf, Jürgen; König, Simone (2006). "Mass analysis sequence prediction (MAPSP)". Bioinformatics. 22 (8): 1002–1003. doi: 10.1093/bioinformatics/btl052 . PMID 16500935.
- ↑ König, Simone; Albers, Christian; Gäde, Gerd (15 November 2005). "Mass spectral signature for insect adipokinetic hormones". Rapid Communications in Mass Spectrometry. 19 (21): 3021–3024. Bibcode:2005RCMS...19.3021K. doi:10.1002/rcm.2167. ISSN 0951-4198. PMID 16193531.
- ↑
- Alavez-Rosas, David; Vargas-Abasolo, Reyna; Albores-Flores, Claudia I.; Meneses-Arias, María Guadalupe; Gutiérrez-Cabrera, Ana Erika; Benelli, Giovanni; Cruz-López, Leopoldo; Córdoba-Aguilar, Alex (2023). "Chemical ecology of triatomines: current knowledge and implications for Chagas disease vector management". Journal of Pest Science . 97 (2). Springer Science and Business Media LLC: 507. Bibcode:2023JPesS..97..507A. doi: 10.1007/s10340-023-01678-6 . ISSN 1612-4758. S2CID 260819708.
- This review cites this research.
- Marco, Heather G.; König, Simone; Gäde, Gerd (January 2022). "Mass Spectrometric Proof of Predicted Peptides: Novel Adipokinetic Hormones in Insects". Molecules. 27 (19): 6469. doi: 10.3390/molecules27196469 . ISSN 1420-3049. PMC 9573411 . PMID 36235010.
- ↑ König, Simone; Bayer, Malte; Marco, Heather; Gäde, Gerd (July 2019). "The hypertrehalosaemic neuropeptide conformational twins of cicadas consist of only l-amino acids: are they cis–trans isomers?". Amino Acids. 51 (7): 1023–1028. doi:10.1007/s00726-019-02742-1. PMID 31073692. S2CID 254074930.
- ↑ Goldsworthy, G., K. Opoku-Ware, and L. Mullen, Adipokinetic hormone enhances laminarin and bacterial lipopolysaccharide-induced activation of the prophenoloxidase cascade in the African migratory locust Locusta migratoria. Journal of Insect Physiology, 2002. 48: p. 601-608
- ↑ Goldsworthy, G.J., K. Opoku-Ware, and L.M. Mullen, Adipokinetic hormone and the immune responses of locusts to infection. Annals of the New York Academy of Science, 2005. 1040: p. 106-113
- ↑ Mugumbate G, Jackson GE, van der Spoel D, Kövér KE, Szilágyi L. Anopheles gambiae, Anoga-HrTH hormone, free and bound structure--a nuclear magnetic resonance experiment. Peptides. 2013 Mar;41:94-100. DOI: 10.1016/j.peptides.2013.01.008.