
The cytochrome complex, or cyt c, is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion where it plays a critical role in cellular respiration. It transfers electrons between Complexes III and IV. Cytochrome c is highly water-soluble, unlike other cytochromes. It is capable of undergoing oxidation and reduction as its iron atom converts between the ferrous and ferric forms, but does not bind oxygen. It also plays a major role in cell apoptosis. In humans, cytochrome c is encoded by the CYCS gene.
Israel Hanukoglu is a Turkish-born Israeli scientist. He is a full professor of biochemistry and molecular biology at Ariel University and former science and technology adviser to the prime minister of Israel (1996–1999). He is founder of Israel Science and Technology Directory.

Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.
Ferredoxins are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.

Rieske proteins are iron–sulfur protein (ISP) components of cytochrome bc1 complexes and cytochrome b6f complexes and are responsible for electron transfer in some biological systems. John S. Rieske and co-workers first discovered the protein and in 1964 isolated an acetylated form of the bovine mitochondrial protein. In 1979 Trumpower's lab isolated the "oxidation factor" from bovine mitochondria and showed it was a reconstitutively-active form of the Rieske iron-sulfur protein
It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. They have since been found in plants, animals, and bacteria with widely ranging electron reduction potentials from -150 to +400 mV.
Any enzyme system that includes cytochrome P450 protein or domain can be called a P450-containing system.
The steroidogenic acute regulatory protein, commonly referred to as StAR (STARD1), is a transport protein that regulates cholesterol transfer within the mitochondria, which is the rate-limiting step in the production of steroid hormones. It is primarily present in steroid-producing cells, including theca cells and luteal cells in the ovary, Leydig cells in the testis and cell types in the adrenal cortex.

Cholesterol side-chain cleavage enzyme is commonly referred to as P450scc, where "scc" is an acronym for side-chain cleavage. P450scc is a mitochondrial enzyme that catalyzes conversion of cholesterol to pregnenolone. This is the first reaction in the process of steroidogenesis in all mammalian tissues that specialize in the production of various steroid hormones.

Steroid 11β-hydroxylase, also known as steroid 11β-monooxygenase, is a steroid hydroxylase found in the zona glomerulosa and zona fasciculata of the adrenal cortex. Named officially the cytochrome P450 11B1, mitochondrial, it is a protein that in humans is encoded by the CYP11B1 gene. The enzyme is involved in the biosynthesis of adrenal corticosteroids by catalyzing the addition of hydroxyl groups during oxidation reactions.

Cytochrome P450 reductase is a membrane-bound enzyme required for electron transfer from NADPH to cytochrome P450 and other heme proteins including heme oxygenase in the endoplasmic reticulum of the eukaryotic cell.
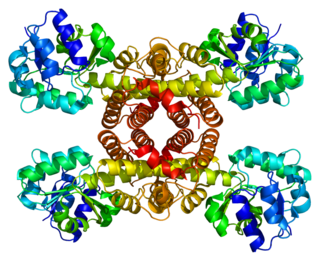
In enzymology, a 3-hydroxyisobutyrate dehydrogenase also known as β-hydroxyisobutyrate dehydrogenase or 3-hydroxyisobutyrate dehydrogenase, mitochondrial (HIBADH) is an enzyme that in humans is encoded by the HIBADH gene.

In enzymology, a NADPH—hemoprotein reductase is an enzyme that catalyzes the chemical reaction
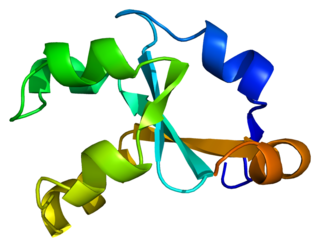
Cytochrome b5, form A, is a human microsomal cytochrome b5.

TYMP is a gene that encodes for the enzyme thymidine phosphorylase. The TYMP gene is also known as ECGF1 and MNGIE due to its role in MNGIE syndrome.

Adrenodoxin reductase, was first isolated from bovine adrenal cortex where it functions as the first enzyme in the mitochondrial P450 systems that catalyze essential steps in steroid hormone biosynthesis. Examination of complete genome sequences revealed that adrenodoxin reductase gene is present in most metazoans and prokaryotes.

Cytochrome b-c1 complex subunit 1, mitochondrial is a protein that in humans is encoded by the UQCRC1 gene.

UQCR11 is a protein that in humans is encoded by the UQCR11 gene. UQCR11 is the smallest known component of Complex III in the mitochondrial respiratory chain.
Flavoprotein pyridine nucleotide cytochrome reductases catalyse the interchange of reducing equivalents between one-electron carriers and the two-electron-carrying nicotinamide dinucleotides. The enzymes include ferredoxin-NADP+ reductases, plant and fungal NAD(P)H:nitrate reductases, cytochrome b5 reductases, cytochrome P450 reductases, sulphite reductases, nitric oxide synthases, phthalate dioxygenase reductase, and various other flavoproteins.
Oxidoreductase NAD-binding domain is an evolutionary conserved protein domain present in a variety of proteins that include, bacterial flavohemoprotein, mammalian NADH-cytochrome b5 reductase, eukaryotic NADPH-cytochrome P450 reductase, nitrate reductase from plants, nitric-oxide synthase, bacterial vanillate demethylase and others.
Walter L. Miller is an American endocrinologist and professor emeritus of pediatrics at the University of California, San Francisco (UCSF). Miller is expert in the field of human steroid biosynthesis and disorders of steroid metabolism. Over the past 40 years Miller's group at UCSF has described molecular basis of several metabolic disorders including, congenital adrenal hyperplasia, pseudo vitamin D dependent rickets, severe, recessive form of Ehlers-Danlos syndrome, 17,20 lyase deficiency caused by CYP17A1 defects, P450scc deficiency caused by CYP11A1 defects, P450 oxidoreductase deficiency.




















