
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.
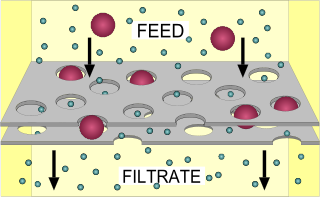
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some chemical, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

A Raschig ring is a piece of tube, approximately equal in length and diameter, used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic, metal, or glass and provide a large surface area within the volume of the column for interaction between liquid and gas vapours.

A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.

Vacuum distillation or distillation under reduced pressure is a type of distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation.
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, azeotropic distillation usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous, such as the example below with the addition of benzene to water and ethanol.

Steam distillation is a separation process that consists of distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container.
Scrubber systems are a diverse group of air pollution control devices that can be used to remove some particulates and/or gases from industrial exhaust streams. An early application of a carbon dioxide scrubber was in the submarine the Ictíneo I, in 1859; a role for which they continue to be used today. Traditionally, the term "scrubber" has referred to pollution control devices that use liquid to wash unwanted pollutants from a gas stream. Recently, the term has also been used to describe systems that inject a dry reagent or slurry into a dirty exhaust stream to "wash out" acid gases. Scrubbers are one of the primary devices that control gaseous emissions, especially acid gases. Scrubbers can also be used for heat recovery from hot gases by flue-gas condensation. They are also used for the high flows in solar, PV, or LED processes.

In chemistry, volatility is a material quality which describes how readily a substance vaporizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate such as dry ice or iodine can vaporize at a similar rate as some liquids under standard conditions.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
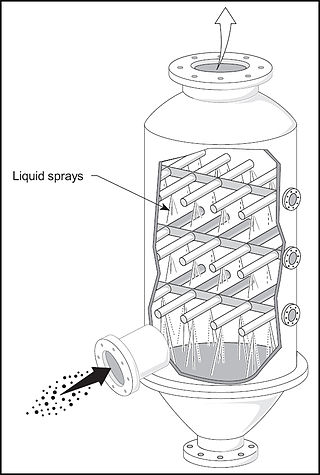
A spray tower is a gas-liquid contactor used to achieve mass and heat transfer between a continuous gas phase and a dispersed liquid phase. It consists of an empty cylindrical vessel made of steel or plastic, and nozzles that spray liquid into the vessel. The inlet gas stream usually enters at the bottom of the tower and moves upward, while the liquid is sprayed downward from one or more levels. This flow of inlet gas and liquid in opposite directions is called countercurrent flow.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.
Stripping is a physical separation process where one or more components are removed from a liquid stream by a vapor stream. In industrial applications the liquid and vapor streams can have co-current or countercurrent flows. Stripping is usually carried out in either a packed or trayed column.

Electrical resistance heating (ERH) is an intensive in situ environmental remediation method that uses the flow of alternating current electricity to heat soil and groundwater and evaporate contaminants. Electric current is passed through a targeted soil volume between subsurface electrode elements. The resistance to electrical flow that exists in the soil causes the formation of heat; resulting in an increase in temperature until the boiling point of water at depth is reached. After reaching this temperature, further energy input causes a phase change, forming steam and removing volatile contaminants. ERH is typically more cost effective when used for treating contaminant source areas.
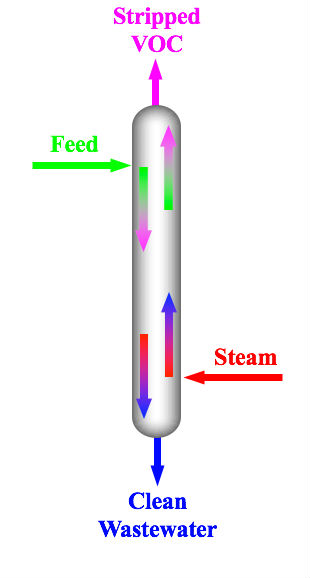
Steam stripping is a process used in petroleum refineries and petrochemical plants to remove volatile contaminants, such as hydrocarbons and other volatile organic compounds (VOCs), from wastewater. It typically consists of passing a stream of superheated steam through the wastewater.

Electro Thermal Dynamic Stripping Process (ET-DSP) is a patented in situ thermal environmental remediation technology, created by McMillan-McGee Corporation, for cleaning contaminated sites. ET-DSP uses readily available three phase electric power to heat the subsurface with electrodes. Electrodes are placed at various depths and locations in the formation. Electric current to each electrode is controlled continuously by computer to uniformly heat the target contamination zone.
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.














