Related Research Articles

Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry.
Sulfur-reducing bacteria are microorganisms able to reduce elemental sulfur (S0) to hydrogen sulfide (H2S). These microbes use inorganic sulfur compounds as electron acceptors to sustain several activities such as respiration, conserving energy and growth, in absence of oxygen. The final product or these processes, sulfide, has a considerable influence on the chemistry of the environment and, in addition, is used as electron donor for a large variety of microbial metabolisms. Several types of bacteria and many non-methanogenic archaea can reduce sulfur. Microbial sulfur reduction was already shown in early studies, which highlighted the first proof of S0 reduction in a vibrioid bacterium from mud, with sulfur as electron acceptor and H2 as electron donor. The first pure cultured species of sulfur-reducing bacteria, Desulfuromonas acetoxidans, was discovered in 1976 and described by Pfennig Norbert and Biebel Hanno as an anaerobic sulfur-reducing and acetate-oxidizing bacterium, not able to reduce sulfate. Only few taxa are true sulfur-reducing bacteria, using sulfur reduction as the only or main catabolic reaction. Normally, they couple this reaction with the oxidation of acetate, succinate or other organic compounds. In general, sulfate-reducing bacteria are able to use both sulfate and elemental sulfur as electron acceptors. Thanks to its abundancy and thermodynamic stability, sulfate is the most studied electron acceptor for anaerobic respiration that involves sulfur compounds. Elemental sulfur, however, is very abundant and important, especially in deep-sea hydrothermal vents, hot springs and other extreme environments, making its isolation more difficult. Some bacteria – such as Proteus, Campylobacter, Pseudomonas and Salmonella – have the ability to reduce sulfur, but can also use oxygen and other terminal electron acceptors.
Paracoccus denitrificans, is a coccoid bacterium known for its nitrate reducing properties, its ability to replicate under conditions of hypergravity and for being a relative of the eukaryotic mitochondrion.
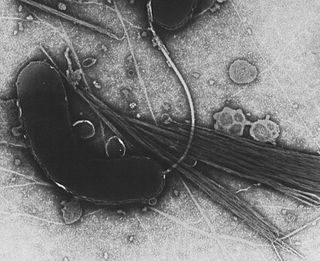
The class Gammaproteobacteria belongs to the Proteobacteria phylum and contains about 250 genera, which makes it the most genera-rich taxon of the Prokaryotes. Several medically, ecologically, and scientifically important groups of bacteria belong to this class. It is composed by all Gram-negative microbes and is the most phylogenetically and physiologically diverse class of Proteobacteria.
Hydrogen oxidizing bacteria are a group of facultative autotrophs that can use hydrogen as an electron donor.
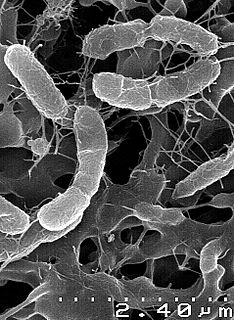
Venenivibrio stagnispumantis strain CP.B2 is the first microorganisms isolated from the terrestrial hot spring Champagne Pool in Waiotapu, New Zealand.

Felisa Wolfe-Simon is an American microbial geobiologist and biogeochemist. In 2010, Wolfe-Simon led a team that discovered GFAJ-1, an extremophile bacterium that they claimed was capable of substituting arsenic for a small percentage of its phosphorus to sustain its growth, thus advancing the remarkable possibility of non-RNA/DNA-based genetics. However, these conclusions were immediately debated and criticized in correspondence to the original journal of publication, and have since come to be widely disbelieved. In 2012, two reports refuting the most significant aspects of the original results were published in the same journal in which the original findings had been previously published.

GFAJ-1 is a strain of rod-shaped bacteria in the family Halomonadaceae. It is an extremophile that was isolated from the hypersaline and alkaline Mono Lake in eastern California by geobiologist Felisa Wolfe-Simon, a NASA research fellow in residence at the US Geological Survey. In a 2010 Science journal publication, the authors claimed that the microbe, when starved of phosphorus, is capable of substituting arsenic for a small percentage of its phosphorus to sustain its growth. Immediately after publication, other microbiologists and biochemists expressed doubt about this claim which was robustly criticized in the scientific community. Subsequent independent studies published in 2012 found no detectable arsenate in the DNA of GFAJ-1, refuted the claim, and demonstrated that GFAJ-1 is simply an arsenate-resistant, phosphate-dependent organism.
Alkalilimnicola is a genus in the phylum Proteobacteria (Bacteria).
Sulfurimonas is a bacterial genus within the class of Epsilonproteobacteria, known for reducing nitrate, oxidizing both sulfur and hydrogen, and containing Group IV hydrogenases. This genus consists of four species: Sulfurimonas autorophica, Sulfurimonas denitrificans, Sulfurimonas gotlandica, and Sulfurimonas paralvinellae. The genus' name is derived from "sulfur" in Latin and "monas" from Greek, together meaning a “sulfur-oxidizing rod”. The size of the bacteria varies between about 1.5-2.5 μm in length and 0.5-1.0 μm in width. Members of the genus Sulfurimonas are found in a variety of different environments which include deep sea-vents, marine sediments, and terrestrial habitats. Their ability to survive in extreme conditions is attributed to multiple copies of one enzyme. Phylogenetic analysis suggests that members of the genus Sulfurimonas have limited dispersal ability and its speciation was affected by geographical isolation rather than hydrothermal composition. Deep ocean currents affect the dispersal of Sulfurimonas spp., influencing its speciation. As shown in the MLSA report of deep-sea hydrothermal vents Epsilonproteobacteria, Sulfurimonas has a higher dispersal capability compared with deep sea hydrothermal vent thermophiles, indicating allopatric speciation.
Variovorax paradoxus is a gram negative, beta proteobacterium from the genus Variovorax. Strains of V. paradoxus can be categorized into two groups, hydrogen oxidizers and heterotrophic strains, both of which are aerobic. The genus name Vario-vorax and species name para-doxus (contrary-opinion) reflects both the dichotomy of V. paradoxus metabolisms, but also its ability to utilize a wide array of organic compounds.
Bacillus selenitireducens is a bacterium first isolated from Mono Lake, California. It is notable for respiring oxyanions of selenium and arsenic. It is spore-forming, rod-shaped and alkaliphile, its type strain being MLS10.
Acidithiobacillus caldus formerly belonged to the genus Thiobacillus prior to 2000, when it was reclassified along with a number of other bacterial species into one of three new genera that better categorize sulfur-oxidizing acidophiles. As a member of the Gammaproteobacteria class of Proteobacteria, A. caldus may be identified as a Gram-negative bacterium that is frequently found in pairs. Considered to be one of the most common microbes involved in biomining, it is capable of oxidizing reduced inorganic sulfur compounds (RISCs) that form during the breakdown of sulfide minerals. The meaning of the prefix acidi- in the name Acidithiobacillus comes from the Latin word acidus, signifying that members of this genus love a sour, acidic environment. Thio is derived from the Greek word thios and describes the use of sulfur as an energy source, and bacillus describes the shape of these microorganisms, which are small rods. The species name, caldus, is derived from the Latin word for warm or hot, denoting this species' love of a warm environment.
Arsenate-reducing bacteria are bacteria which reduce arsenates. Arsenate-reducing bacteria are ubiquitous in arsenic-contaminated groundwater (aqueous environment). Arsenates are salts or esters of arsenic acid (H3AsO4), consisting of the ion AsO43−. They are moderate oxidizers that can be reduced to arsenites and to arsine. Arsenate can serve as a respiratory electron acceptor for oxidation of organic substrates and H2S or H2. Arsenates occur naturally in minerals such as adamite, alarsite, legrandite, and erythrite, and as hydrated or anhydrous arsenates. Arsenates are similar to phosphates since arsenic (As) and phosphorus (P) occur in group 15 (or VA) of the periodic table. Unlike phosphates, arsenates are not readily lost from minerals due to weathering. They are the predominant form of inorganic arsenic in aqueous aerobic environments. On the other hand, arsenite is more common in anaerobic environments, more mobile, and more toxic than arsenate. Arsenite is 25–60 times more toxic and more mobile than arsenate under most environmental conditions. Arsenate can lead to poisoning, since it can replace inorganic phosphate in the glyceraldehyde-3-phosphate --> 1,3-biphosphoglycerate step of glycolysis, producing 1-arseno-3-phosphoglycerate instead. Although glycolysis continues, 1 ATP molecule is lost. Thus, arsenate is toxic due to its ability to uncouple glycolysis. Arsenate can also inhibit pyruvate conversion into acetyl-CoA, thereby blocking the TCA cycle, resulting in additional loss of ATP.
Rhodoferax is a genus of Betaproteobacteria belonging to the purple nonsulfur bacteriarophic. Originally, Rhodoferax species were included in the genus Rhodocyclus as the Rhodocyclus gelatinous-like group. The genus Rhodoferax was first proposed in 1991 to accommodate the taxonomic and phylogenetic discrepancies arising from its inclusion in the genus Rhodocyclus. Rhodoferax currently comprises four described species: R. fermentans, R. antarcticus, R. ferrireducens, and R. saidenbachensis. R. ferrireducens, lacks the typical phototrophic character common to two other Rhodoferax species. This difference has led researchers to propose the creation of a new genus, Albidoferax, to accommodate this divergent species. The genus name was later corrected to Albidiferax. Based on geno- and phenotypical characteristics, A. ferrireducens was reclassified in the genus Rhodoferax in 2014. R. saidenbachensis, a second non-phototrophic species of the genus Rhodoferax was described by Kaden et al. in 2014.
Thioalkalivibrio versutus is an obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria. It was first isolated from soda lakes in northern Russia.
Thalassolituus oleivorans is a species of bacteria, the type species of its genus. It is an aerobic, heterotrophic, Gram-negative, curved bacteria that metabolises aliphatic hydrocarbons, their oxidized derivatives and acetate, with type strain MIL-1T.
Desulfonatronum thiodismutans is an alkaliphilic, sulfate-reducing bacterium capable of lithoautotrophic growth. It is Gram-negative, vibrio-shaped, with cells 0.6–0.7×1.2–2.7 μm in size, motile by a single polar flagellum. Its type strain is MLF1T.
Thioalkalivibrio is a Gram-negative, mostly halophilic bacterial genus of the family Ectothiorhodospiraceae.
Acidithrix ferrooxidans is a heterotrophic, acidophilic and Gram-positive bacterium from the genus of Acidithrix. The type strain of this species, A. ferrooxidans Py-F3 was isolated from an acidic stream draining from a copper mine in Wales. This species grows in a variety of acidic environments such as streams, mines or geothermal sites. Mine lakes with a redoxcline support growth with ferrous iron as the electron donor. A. ferrooxidans grows rapidly in macroscopic streamer, producing greater cell densities than other streamer-forming microbes. Use in a bioreactors to remediate mine waste has been proposed due to cell densities and rapid oxidation of ferrous iron oxidation in acidic mine drainage. Exopolysaccharide production during metal substrate metabolism, such as iron oxidation helps to prevent cell encrustation by minerals.
References
- ↑ Hoeft, S. E.; Blum, J. S.; Stolz, J. F.; Tabita, F. R.; Witte, B.; King, G. M.; Santini, J. M.; Oremland, R. S. (2007). "Alkalilimnicola ehrlichii sp. nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor". International Journal of Systematic and Evolutionary Microbiology. 57 (3): 504–512. doi: 10.1099/ijs.0.64576-0 . ISSN 1466-5026. PMID 17329775.