
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.

Joseph Leonard Goldstein ForMemRS is an American biochemist. He received the Nobel Prize in Physiology or Medicine in 1985, along with fellow University of Texas Southwestern researcher, Michael Brown, for their studies regarding cholesterol. They discovered that human cells have low-density lipoprotein (LDL) receptors that remove cholesterol from the blood and that when LDL receptors are not present in sufficient numbers, individuals develop hypercholesterolemia and become at risk for cholesterol related diseases, notably coronary heart disease. Their studies led to the development of statin drugs.

Thymosins are small proteins present in many animal tissues. They are named thymosins because they were originally isolated from the thymus, but most are now known to be present in many other tissues. Thymosins have diverse biological activities, and two in particular, thymosins α1 and β4, have potentially important uses in medicine, some of which have already progressed from the laboratory to the clinic. In relation to diseases, thymosins have been categorized as biological response modifiers. Thymosins are important for proper T-cell development and differentiation.
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.

Imiquimod, sold under the brand name Aldara among others, is a medication that acts as an immune response modifier that is used to treat genital warts, superficial basal cell carcinoma, and actinic keratosis. Scientists at 3M's pharmaceuticals division discovered the drug and 3M obtained the first FDA approval in 1997. As of 2015, imiquimod is generic and is available worldwide under many brands.
Neal A. Halsey is an American pediatrician, with sub-specialty training in infectious diseases, international health and epidemiology. Halsey is a professor emeritus of international health and director emeritus of the Institute for Vaccine Safety at the Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland. He had a joint appointment in the Department of Pediatrics at the Johns Hopkins School of Medicine and serves as co-director of the Center for Disease Studies and Control in Guatemala.
Biological response modifiers (BRMs) are substances that modify immune responses. They can be both endogenous and exogenous, and they can either enhance an immune response or suppress it. Some of these substances arouse the body's response to an infection, and others can keep the response from becoming excessive. Thus they serve as immunomodulators in immunotherapy, which can be helpful in treating cancer and in treating autoimmune diseases, such as some kinds of arthritis and dermatitis. Most BRMs are biopharmaceuticals (biologics), including monoclonal antibodies, interleukin 2, interferons, and various types of colony-stimulating factors. "Immunotherapy makes use of BRMs to enhance the activity of the immune system to increase the body's natural defense mechanisms against cancer", whereas BRMs for rheumatoid arthritis aim to reduce inflammation.

Thymosin beta-4 is a protein that in humans is encoded by the TMSB4X gene. Recommended INN for thymosin beta-4 is 'timbetasin', as published by the World Health Organization (WHO).
Vedolizumab, sold under the brand name Entyvio, is a monoclonal antibody medication developed by Millennium Pharmaceuticals, Inc. for the treatment of ulcerative colitis and Crohn's disease. It binds to integrin α4β7. Blocking the α4β7 integrin results in gut-selective anti-inflammatory activity.
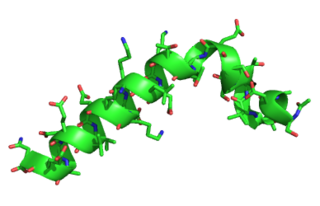
Thymosin α1 is a peptide fragment derived from prothymosin alpha, a protein that in humans is encoded by the PTMA gene.
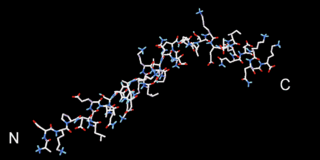
Beta thymosins are a family of proteins which have in common a sequence of about 40 amino acids similar to the small protein thymosin β4. They are found almost exclusively in multicellular animals. Thymosin β4 was originally obtained from the thymus in company with several other small proteins which although named collectively "thymosins" are now known to be structurally and genetically unrelated and present in many different animal tissues.
Molecular oncology is an interdisciplinary medical specialty at the interface of medicinal chemistry and oncology that refers to the investigation of the chemistry of cancer and tumors at the molecular scale. Also the development and application of molecularly targeted therapies.
Tildrakizumab, sold under the brand names Ilumya and Ilumetri, is a monoclonal antibody designed for the treatment of immunologically mediated inflammatory disorders. It is approved for the treatment of adult patients with moderate-to-severe plaque psoriasis in the United States and the European Union.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

David A. Hafler is an American neurologist. He is the Edgerly Professor and chairman of the department of Neurology at the Yale School of Medicine. He is known for his work in immunity, genetics, and multiple sclerosis. In 2018 he was elected to the National Academy of Medicine.
Katherine A. High is an American doctor-scientist who is an emeritus professor at the Perelman School of Medicine at the University of Pennsylvania. She was the co-founder, president, and chief scientific officer of Spark Therapeutics and currently serves as President of Therapeutics at AskBio. Her career has focused on pioneering work in the area of gene therapy, with many accomplishments in basic, translational, and clinical investigation in gene therapy.
Satralizumab, sold under the brand name Enspryng, is a humanized monoclonal antibody medication that is used for the treatment of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disease. The drug is being developed by Chugai Pharmaceutical, a subsidiary of Roche.

SCB-2019 is a protein subunit COVID-19 vaccine developed by Clover Biopharmaceuticals using an adjuvant from Dynavax technologies. Positive results of Phase I trials for the vaccine were published in The Lancet and the vaccine completed enrollment of 29,000 participants in Phase II/III trials in July 2021. In September 2021, SCB-2019 announced Phase III results showing 67% efficacy against all cases of COVID-19 and 79% efficacy against all cases of the Delta variant. Additionally, the vaccine was 84% effective against moderate cases and 100% effective against hospitalization.










