
An allyl group is a substituent with the structural formula H2C=CH−CH2R, where R is the rest of the molecule. It consists of a methylene bridge (−CH2−) attached to a vinyl group (−CH=CH2). The name is derived from the Latin word for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
The Carroll rearrangement is a rearrangement reaction in organic chemistry and involves the transformation of a β-keto allyl ester into a α-allyl-β-ketocarboxylic acid. This organic reaction is accompanied by decarboxylation and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement and effectively a decarboxylative Allylation.

Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is also used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Methohexital or methohexitone is a drug which is a barbiturate derivative. It is classified as short-acting, and has a rapid onset of action. It is similar in its effects to sodium thiopental, a drug with which it competed in the market for anaesthetics.
Phenylacetate may refer to:
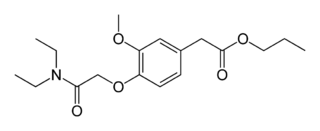
Propanidid is an ultra short-acting phenylacetate general anesthetic. It was originally introduced by Bayer in 1963 but anaphylactic reactions caused it to be withdrawn shortly afterwards.

A boronic acid is a compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc.. The pKa of a boronic acid is ~9, but they can form tetrahedral boronate complexes with pKa ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
In enzymology, a (S)-mandelate dehydrogenase is an enzyme that catalyzes the chemical reaction

Olfactory receptor 2W1 is a protein that in humans is encoded by the OR2W1 gene.

Olfactory receptor 51L1 is a protein that in humans is encoded by the OR51L1 gene.

Methyl phenylacetate is an organic compound that is the methyl ester of phenylacetic acid, with the structural formula C6H5CH2COOCH3. It is a colorless liquid that is only slightly soluble in water, but soluble in most organic solvents.
The molecular formula C11H12O2 may refer to:
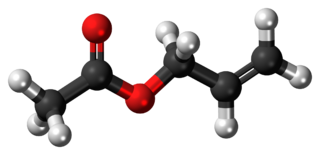
Allyl acetate is an organic compound with formula C3H5OC(O)CH3. This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate. It is the acetate ester of allyl alcohol.
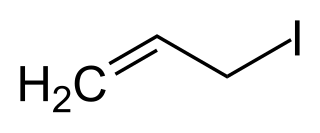
Allyl iodide (3-iodopropene) is an organic halide used in synthesis of other organic compounds such as N-alkyl 2-pyrrolidones, sorbic acid esters, 5,5-disubstituted barbituric acids, and organometallic catalysts. Allyl iodide can be synthesized from allyl alcohol and methyl iodide on triphenyl phosphite, Finkelstein reaction on allyl halides, or by the action of elemental phosphorus and iodine on glycerol. Allyl iodide dissolved in hexane can be stored for up to three months in a dark freezer at −5 °C (23 °F) before decomposition into free iodine becomes apparent.

Testosterone phenylacetate is an androgen and anabolic steroid and a testosterone ester. Analogously to estradiol benzoate having been one of the first estrogen esters to be introduced, testosterone phenylacetate was one of the first testosterone esters to be introduced. However, since its introduction, it has largely been replaced by other esters, such as testosterone propionate.
Phenestrol, or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard ester of hexestrol which was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.
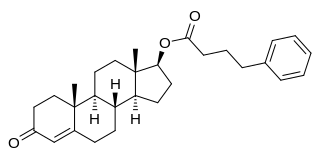
Testosterone phenylbutyrate, also known as testosterone phenylbutanoate, testosterone 17β-phenylbutyrate, and androst-4-en-17β-ol-3-one 17β-phenylbutyrate, is a synthetic, injected anabolic-androgenic steroid (AAS) and an androgen ester – specifically, the C17β phenylbutyrate (phenylbutanoate) ester of testosterone – which was never marketed. It is a prodrug of testosterone and, when administered via intramuscular injection, is associated with a long-lasting depot effect and extended duration of action.














