In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

In organic chemistry, a carbonyl group is a functional group with the formula C=O, composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.

An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties react to form a β-hydroxyaldehyde or β-hydroxyketone, and this is then followed by dehydration to give a conjugated enone.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
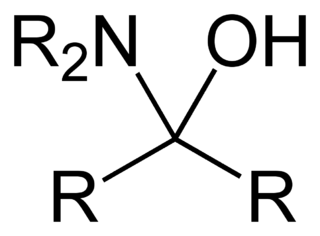
In organic chemistry, a hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: −C(OH)(NR2)−. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution. Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols.
![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula [SR3]+. Together with a negatively-charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in organic solvent.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.

Hydroperoxides or peroxols are compounds of the form ROOH, which contain the hydroperoxy functional group (–OOH). The hydroperoxide anion and the neutral hydroperoxyl radical (HOO·) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.

Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals.

In organic chemistry, the sulfonamide functional group is an organosulfur group with the structure R−S(=O)2−NR2. It consists of a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.

In chemistry, ureas are a class of organic compounds with the formula (R2N)2CO where R = H, alkyl, aryl, etc. Thus, in addition to describing the specific chemical compound urea ((H2N)2CO), urea is the name of a functional group that is found in many compounds and materials of both practical and theoretical interest. Generally ureas are colorless crystalline solids, which, owing to the presence of fewer hydrogen bonds, exhibit melting points lower than that of urea itself.
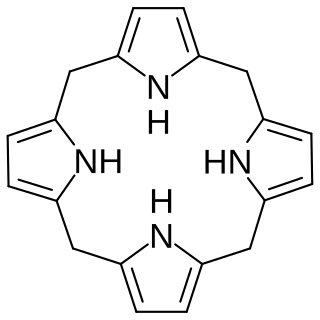
In biochemistry a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle of four pyrrole rings connected by four methylene bridges. They can be viewed as derived from the parent compound hexahydroporphine by the substitution of various functional groups for hydrogen atoms in the outermost (20-carbon) ring.
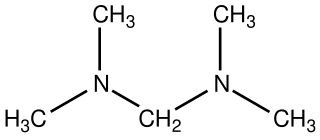
Bis(dimethylamino)methane is the organic compound with the formula [(CH3)2N]2CH2. It is classified as an aminal as well as a ditertiary amine, in fact the simplest. It is a colorless liquid that is widely available. It is prepared by the reaction of dimethylamine and formaldehyde:












![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)





