Contents
- Biosynthetic origins
- Biosynthesis pathway
- Chemical structure and proposed mechanism of formation
- Pharmaceutical potential
- Synthetic preparation
- References
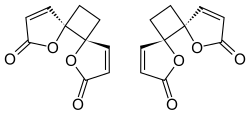 | |
 | |
| Names | |
|---|---|
| IUPAC names trans-4,7-Dioxadispiro[4.0.46.25]dodeca-1,9-diene-3,8-dione trans-1,7-Dioxadispiro[4.0.4.2]dodeca-3,9-diene-2,8-dione [1] | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H8O4 | |
| Molar mass | 192.170 g·mol−1 |
| Appearance | Colourless, odourless solid |
| Density | 1.45g/cm3 |
| Melting point | 158 [1] °C (316 °F; 431 K) |
| Boiling point | 535.7 °C (996.3 °F; 808.9 K) @ 760mmHg |
| low | |
| Solubility in chloroform | very soluble [1] |
| Hazards | |
| Flash point | 300.7 °C (573.3 °F; 573.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 150 mg·kg−1 (mouse, IP) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Anemonin is a dibutenolide natural product found in members of the buttercup family (Ranunculaceae) such as Helleborus niger , Ranunculus bulbosus , R. ficaria , R. sardous , R. sceleratus , [2] and Clematis hirsutissima . [3] Originally isolated in 1792 by M. Heyer, [4] It is the dimerization product of the toxin protoanemonin. [5] One of the likely active agents in plants used in Chinese medicine as an anti-inflammatory [6] and Native American medicine as a horse stimulant, [3] its unique biological properties give it pharmaceutical potential as an anti-inflammatory agent.




