Related Research Articles

A saccade is a quick, simultaneous movement of both eyes between two or more phases of fixation in the same direction. In contrast, in smooth pursuit movements, the eyes move smoothly instead of in jumps. The phenomenon can be associated with a shift in frequency of an emitted signal or a movement of a body part or device. Controlled cortically by the frontal eye fields (FEF), or subcortically by the superior colliculus, saccades serve as a mechanism for fixation, rapid eye movement, and the fast phase of optokinetic nystagmus. The word appears to have been coined in the 1880s by French ophthalmologist Émile Javal, who used a mirror on one side of a page to observe eye movement in silent reading, and found that it involves a succession of discontinuous individual movements.

In the human brain, the anterior cingulate cortex (ACC) is the frontal part of the cingulate cortex that resembles a "collar" surrounding the frontal part of the corpus callosum. It consists of Brodmann areas 24, 32, and 33.

The frontal lobe is the largest of the four major lobes of the brain in mammals, and is located at the front of each cerebral hemisphere. It is parted from the parietal lobe by a groove between tissues called the central sulcus and from the temporal lobe by a deeper groove called the lateral sulcus. The most anterior rounded part of the frontal lobe is known as the frontal pole, one of the three poles of the cerebrum.
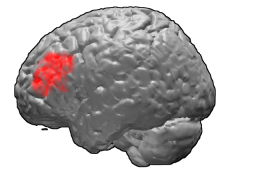
Brodmann area 46, or BA46, is part of the frontal cortex in the human brain. It is between BA10 and BA45.

Eye movement includes the voluntary or involuntary movement of the eyes. Eye movements are used by a number of organisms to fixate, inspect and track visual objects of interests. A special type of eye movement, rapid eye movement, occurs during REM sleep.
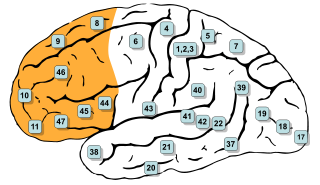
In mammalian brain anatomy, the prefrontal cortex (PFC) covers the front part of the frontal lobe of the cerebral cortex. The PFC contains the Brodmann areas BA8, BA9, BA10, BA11, BA12, BA13, BA14, BA24, BA25, BA32, BA44, BA45, BA46, and BA47.

In the scientific study of vision, smooth pursuit describes a type of eye movement in which the eyes remain fixated on a moving object. It is one of two ways that visual animals can voluntarily shift gaze, the other being saccadic eye movements. Pursuit differs from the vestibulo-ocular reflex, which only occurs during movements of the head and serves to stabilize gaze on a stationary object. Most people are unable to initiate pursuit without a moving visual signal. The pursuit of targets moving with velocities of greater than 30°/s tends to require catch-up saccades. Smooth pursuit is asymmetric: most humans and primates tend to be better at horizontal than vertical smooth pursuit, as defined by their ability to pursue smoothly without making catch-up saccades. Most humans are also better at downward than upward pursuit. Pursuit is modified by ongoing visual feedback.
Frontal lobe epilepsy (FLE) is a neurological disorder that is characterized by brief, recurring seizures arising in the frontal lobes of the brain, that often occur during sleep. It is the second most common type of epilepsy after temporal lobe epilepsy (TLE), and is related to the temporal form in that both forms are characterized by partial (focal) seizures.

In cognitive science and neuropsychology, executive functions are a set of cognitive processes that are necessary for the cognitive control of behavior: selecting and successfully monitoring behaviors that facilitate the attainment of chosen goals. Executive functions include basic cognitive processes such as attentional control, cognitive inhibition, inhibitory control, working memory, and cognitive flexibility. Higher-order executive functions require the simultaneous use of multiple basic executive functions and include planning and fluid intelligence.

Frontal lobe disorder, also frontal lobe syndrome, is an impairment of the frontal lobe of the brain due to disease or frontal lobe injury. The frontal lobe plays a key role in executive functions such as motivation, planning, social behaviour, and speech production. Frontal lobe syndrome can be caused by a range of conditions including head trauma, tumours, neurodegenerative diseases, neurodevelopmental disorders, neurosurgery and cerebrovascular disease. Frontal lobe impairment can be detected by recognition of typical signs and symptoms, use of simple screening tests, and specialist neurological testing.

The frontal eye fields (FEF) are a region located in the frontal cortex, more specifically in Brodmann area 8 or BA8, of the primate brain. In humans, it can be more accurately said to lie in a region around the intersection of the middle frontal gyrus with the precentral gyrus, consisting of a frontal and parietal portion. The FEF is responsible for saccadic eye movements for the purpose of visual field perception and awareness, as well as for voluntary eye movement. The FEF communicates with extraocular muscles indirectly via the paramedian pontine reticular formation. Destruction of the FEF causes deviation of the eyes to the ipsilateral side.

Supplementary eye field (SEF) is the name for the anatomical area of the dorsal medial frontal lobe of the primate cerebral cortex that is indirectly involved in the control of saccadic eye movements. Evidence for a supplementary eye field was first shown by Schlag, and Schlag-Rey. Current research strives to explore the SEF's contribution to visual search and its role in visual salience. The SEF constitutes together with the frontal eye fields (FEF), the intraparietal sulcus (IPS), and the superior colliculus (SC) one of the most important brain areas involved in the generation and control of eye movements, particularly in the direction contralateral to their location. Its precise function is not yet fully known. Neural recordings in the SEF show signals related to both vision and saccades somewhat like the frontal eye fields and superior colliculus, but currently most investigators think that the SEF has a special role in high level aspects of saccade control, like complex spatial transformations, learned transformations, and executive cognitive functions.
Attentional shift occurs when directing attention to a point increases the efficiency of processing of that point and includes inhibition to decrease attentional resources to unwanted or irrelevant inputs. Shifting of attention is needed to allocate attentional resources to more efficiently process information from a stimulus. Research has shown that when an object or area is attended, processing operates more efficiently. Task switching costs occur when performance on a task suffers due to the increased effort added in shifting attention. There are competing theories that attempt to explain why and how attention is shifted as well as how attention is moved through space.
Frontostriatal circuits are neural pathways that connect frontal lobe regions with the basal ganglia (striatum) that mediate motor, cognitive, and behavioural functions within the brain. They receive inputs from dopaminergic, serotonergic, noradrenergic, and cholinergic cell groups that modulate information processing. Frontostriatal circuits are part of the executive functions. Executive functions include the following: selection and perception of important information, manipulation of information in working memory, planning and organization, behavioral control, adaptation to changes, and decision making. These circuits are involved in neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease as well as neuropsychiatric disorders including schizophrenia, depression, obsessive compulsive disorder (OCD), and in neurodevelopmental disorder such as attention-deficit hyperactivity disorder (ADHD).

The medial dorsal nucleus is a large nucleus in the thalamus.

The posterior parietal cortex plays an important role in planned movements, spatial reasoning, and attention.
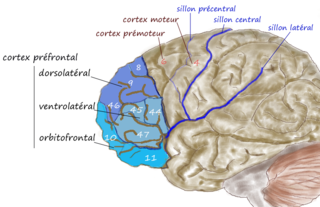
The dorsolateral prefrontal cortex is an area in the prefrontal cortex of the primate brain. It is one of the most recently derived parts of the human brain. It undergoes a prolonged period of maturation which lasts into adulthood. The DLPFC is not an anatomical structure, but rather a functional one. It lies in the middle frontal gyrus of humans. In macaque monkeys, it is around the principal sulcus. Other sources consider that DLPFC is attributed anatomically to BA 9 and 46 and BA 8, 9 and 10.

The frontal lobe of the human brain is both relatively large in mass and less restricted in movement than the posterior portion of the brain. It is a component of the cerebral system, which supports goal directed behavior. This lobe is often cited as the part of the brain responsible for the ability to decide between good and bad choices, as well as recognize the consequences of different actions. Because of its location in the anterior part of the head, the frontal lobe is arguably more susceptible to injuries. Following a frontal lobe injury, an individual's abilities to make good choices and recognize consequences are often impaired. Memory impairment is another common effect associated with frontal lobe injuries, but this effect is less documented and may or may not be the result of flawed testing. Damage to the frontal lobe can cause increased irritability, which may include a change in mood and an inability to regulate behavior. Particularly, an injury of the frontal lobe could lead to deficits in executive function, such as anticipation, goal selection, planning, initiation, sequencing, monitoring, and self-correction. A widely reported case of frontal lobe injury was that of Phineas Gage, a railroad worker whose left frontal lobe was damaged by a large iron rod in 1848.

Visual processing abnormalities in schizophrenia are commonly found, and contribute to poor social function.
Visual spatial attention is a form of visual attention that involves directing attention to a location in space. Similar to its temporal counterpart visual temporal attention, these attention modules have been widely implemented in video analytics in computer vision to provide enhanced performance and human interpretable explanation of deep learning models.
References
- 1 2 3 4 Levy, D.; Mendell, N.; Holzman, P. (2004). "The antisaccade task and neuropsychological tests of prefrontal cortical integrity in schizophrenia: empirical findings and interpretative considerations". World Psychiatry. 3 (1): 32–40. PMC 1414662 . PMID 16633452.
- ↑ O'Driscoll, G.; Alpert, N.; Mathysse, S.; Levy, S.; Rauch, S.; Holzmann, P. (1995). "Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomorgraphy". Proceedings of the National Academy of Sciences. 92 (3): 925–929. Bibcode:1995PNAS...92..925O. doi: 10.1073/pnas.92.3.925 . PMC 42733 . PMID 7846080.
- ↑ Kissler, Johanna, and Andreas Keil. "Look–don’t look! How emotional pictures affect pro-and anti-saccades." Experimental Brain Research 188.2 (2008): 215-222.
- ↑ Deuter, C. E.; Schilling, T. M.; Kuehl, L. K.; Blumenthal, T. D.; Schachinger, H. (2013). "Startle effects on saccadic responses to emotional target stimuli". Psychophysiology. 50 (10): 1056–1063. doi:10.1111/psyp.12083. PMID 23841560.
- ↑ Ainsworth, B.; Garner, M. (2013). "Attention control in mood and anxiety disorders: evidence from the antisaccade task". Human Psychopharmacology: Clinical and Experimental. 28 (3): 274–280. doi:10.1002/hup.2320. PMID 23653434. S2CID 42743016.
- ↑ Wachter, N. J.; Gilbert, D. G. (2013). "Nicotine differentially modulates antisaccade eye-gaze away from emotional stimuli in nonsmokers stratified by pre-task baseline performance". Psychopharmacology. 225 (3): 561–568. doi:10.1007/s00213-012-2842-6. PMC 3547148 . PMID 22955567.
- ↑ Wieser, M. J.; Pauli, P.; Mühlberger, A. (2009). "Probing the attentional control theory in social anxiety: An emotional saccade task". Cognitive, Affective, & Behavioral Neuroscience. 9 (3): 314–322. doi: 10.3758/cabn.9.3.314 . PMID 19679766.
- ↑ Hallett, P. E. (1978). "Primary and secondary saccades to goals defined by instructions". Vision Research. 18 (10): 1279–1296. doi:10.1016/0042-6989(78)90218-3. PMID 726270. S2CID 27735329.
- ↑ Guitton, D; Buchtel, HA; Douglas, RM (1985). "Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades" (PDF). Exp Brain Res. 58 (3): 455–472. doi:10.1007/bf00235863. hdl: 2027.42/46554 . PMID 4007089. S2CID 10551663.
- ↑ Pierrot-Deseilligny, C; Rivaud, S; Gaymard, B; et al. (1991). "Cortical control of reflexive visually-guided saccades". Brain. 114 (3): 1473–1485. doi:10.1093/brain/114.3.1473. PMID 2065261.