
A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
The Friedländer synthesis is a chemical reaction of 2-aminobenzaldehydes with ketones to form quinoline derivatives. It is named after German chemist Paul Friedländer (1857–1923).

Prodigiosin is the red dyestuff produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginines family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.
Martin Gerhardt Banwell, Hon.FRSNZ is an organic chemist specialising in biotransformations and natural product synthesis.

Triflusal is a platelet aggregation inhibitor that was discovered and developed in the Uriach Laboratories, and commercialised in Spain since 1981. Currently, it is available in 25 countries in Europe, Asia, Africa and America. It is a derivative of acetylsalicylic acid (ASA) in which a hydrogen atom on the benzene ring has been replaced by a trifluromethyl group. Trade names include Disgren, Grendis, Aflen and Triflux.

Ceramide glucosyltransferase is an enzyme that in humans is encoded by the UGCG gene.

60S ribosomal protein L23 is a protein that in humans is encoded by the RPL23 gene.

Hyaluronan synthase 3 is an enzyme that in humans is encoded by the HAS3 gene.

High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A is an enzyme that in humans is encoded by the PDE7A gene. Mammals possess 21 cyclic nucleotide phosphodiesterase (PDE) genes that are pharmacologically grouped into 11 families. PDE7A is one of two genes in the PDE7 family, the other being PDE7B. The PDE7 family, along with the PDE4 and PDE8 families, are cAMP-specific, showing little to no activity against 3', 5'-cyclic guanosine monophosphate (cGMP).

Indole is an aromatic heterocyclic organic compound with the formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.

Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.

Pillararenes are macrocycles composed of hydroquinone or dialkoxybenzene units linked in the para position by methylene bridges. They are structurally similar to the cucurbiturils and calixarenes that play an important part in host–guest chemistry. The first pillararene was the five membered dimethoxypillar[5]arene.

HU-320 (7-nor-7-carboxy-CBD-1,1-DMH) is a drug related to cannabidiol, which has strong antiinflammatory and immunosuppressive properties while demonstrating no psychoactive effects.

Taiwanofungus camphoratus, also known as stout camphor fungus, is a species of fungus that is endemic to Taiwan, where it grows only on the endemic tree Cinnamomum kanehirae, causing a brown heart rot. Synonyms include Antrodia camphorata and Ganoderma camphoratum.
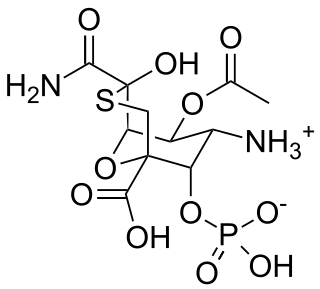
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.

Aspidospermidine is an alkaloid isolated from plants in the genus Aspidosperma. It has been a popular target for total synthesis, due in part to the fact that it provides a good showcase for synthetic strategies but also because the structure is similar to many other important bioactive molecules.

Diverted total synthesis in chemistry is a strategy in drug discovery aiming at organic synthesis of natural product analogues rather than the natural product itself. The target can be the modification of a natural product or the modification of an intermediate. In this sense it differs from other strategies such as total synthesis and semisynthesis. The purpose can be gaining a scientific understanding of the biological activity of the original natural product or the discovery of new drugs with the same biological activity but simpler to produce. The concept was introduced by Samuel J. Danishefsky in 2006. Notable examples of this strategy are the potential drug ixabepilone which is an analogue of the natural product epothilone B and carfilzomib which is derived from epoxomicin and eravacycline derived from tetracycline. Cabergoline is derived from a number of ergot alkaloids one of which is lysergic acid and Simvastatin is based on Lovastatin.

9-Methyl-β-carboline (9-Me-BC) is a heterocyclic amine of the β-carboline family, and a research chemical.


















