Related Research Articles

The entorhinal cortex (EC) is an area of the brain's allocortex, located in the medial temporal lobe, whose functions include being a widespread network hub for memory, navigation, and the perception of time. The EC is the main interface between the hippocampus and neocortex. The EC-hippocampus system plays an important role in declarative (autobiographical/episodic/semantic) memories and in particular spatial memories including memory formation, memory consolidation, and memory optimization in sleep. The EC is also responsible for the pre-processing (familiarity) of the input signals in the reflex nictitating membrane response of classical trace conditioning; the association of impulses from the eye and the ear occurs in the entorhinal cortex.
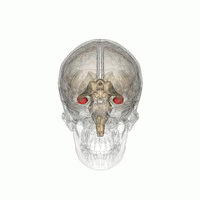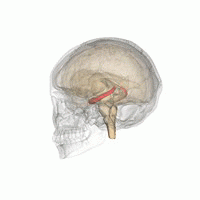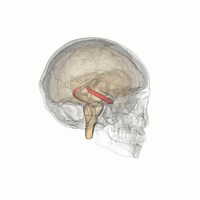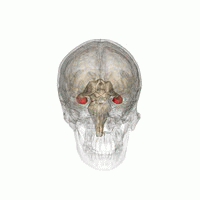
The hippocampus is a major component of the brain of humans and other vertebrates. Humans and other mammals have two hippocampi, one in each side of the brain. The hippocampus is part of the limbic system, and plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus is located in the allocortex, with neural projections into the neocortex in humans, as well as primates. The hippocampus, as the medial pallium, is a structure found in all vertebrates. In humans, it contains two main interlocking parts: the hippocampus proper, and the dentate gyrus.

The limbic system, also known as the paleomammalian cortex, is a set of brain structures located on both sides of the thalamus, immediately beneath the medial temporal lobe of the cerebrum primarily in the forebrain.
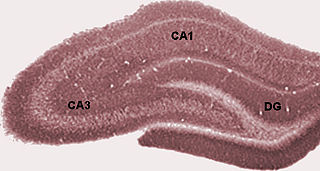
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions.

Adult neurogenesis is the process in which neurons are generated from neural stem cells in the adult. This process differs from prenatal neurogenesis.
The entorhinal cortex (EC) is a major part of the hippocampal formation of the brain, and is reciprocally connected with the hippocampus.

Temporal lobe epilepsy (TLE) is a chronic disorder of the nervous system which is characterized by recurrent, unprovoked focal seizures that originate in the temporal lobe of the brain and last about one or two minutes. TLE is the most common form of epilepsy with focal seizures. A focal seizure in the temporal lobe may spread to other areas in the brain when it may become a focal to bilateral seizure.

In the brain, the perforant path or perforant pathway provides a connectional route from the entorhinal cortex to all fields of the hippocampal formation, including the dentate gyrus, all CA fields, and the subiculum.
Brian R. Christie is a Professor of Medicine and Neuroscience at The University of Victoria. He helped found the Neuroscience Graduate Program at the University of Victoria and served as its director from 2010–2017. He is a Michael Smith Senior Scholar Award winner. Christie received his PhD in 1992 from the University of Otago before doing postdoctoral work with Daniel Johnston at Baylor College of Medicine and Terrence Sejnowski at the Salk Institute for Biological Studies, and then became Assistant Professor at the University of British Columbia. Promoted to Associate Professor in 2007. Full Professor in 2013.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.
The trisynaptic circuit, or trisynaptic loop is a relay of synaptic transmission in the hippocampus. The circuit was initially described by the neuroanatomist Santiago Ramon y Cajal, in the early twentieth century, using the Golgi staining method. After the discovery of the trisynaptic circuit, a series of research has been conducted to determine the mechanisms driving this circuit. Today, research is focused on how this loop interacts with other parts of the brain, and how it influences human physiology and behaviour. For example, it has been shown that disruptions within the trisynaptic circuit leads to behavioural changes in rodent and feline models.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.
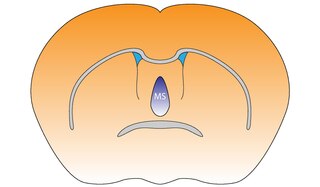
The medial septal nucleus (MS) is one of the septal nuclei. Neurons in this nucleus give rise to the bulk of efferents from the septal nuclei. A major projection from the medial septal nucleus terminates in the hippocampal formation.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.

The hippocampus is an area of the brain integral to learning and memory. Removal of this structure can result in the inability to form new memories as most famously demonstrated in a patient referred to as HM. The unique morphology of the hippocampus can be appreciated without the use of special stains and this distinct circuitry has helped further the understanding of neuronal signal potentiation. The following will provide an introduction to hippocampal development with particular focus on the role of glucocorticoid signaling.
The hippocampus participates in the encoding, consolidation, and retrieval of memories. The hippocampus is located in the medial temporal lobe (subcortical), and is an infolding of the medial temporal cortex. The hippocampus plays an important role in the transfer of information from short-term memory to long-term memory during encoding and retrieval stages. These stages do not need to occur successively, but are, as studies seem to indicate, and they are broadly divided in the neuronal mechanisms that they require or even in the hippocampal areas that they seem to activate. According to Gazzaniga, "encoding is the processing of incoming information that creates memory traces to be stored." There are two steps to the encoding process: "acquisition" and "consolidation". During the acquisition process, stimuli are committed to short term memory. Then, consolidation is where the hippocampus along with other cortical structures stabilize an object within long term memory, which strengthens over time, and is a process for which a number of theories have arisen to explain the underlying mechanism. After encoding, the hippocampus is capable of going through the retrieval process. The retrieval process consists of accessing stored information; this allows learned behaviors to experience conscious depiction and execution. Encoding and retrieval are both affected by neurodegenerative and anxiety disorders and epilepsy.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
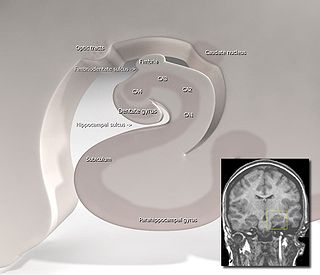
The hippocampus proper refers to the actual structure of the hippocampus which is made up of three regions or subfields. The subfields CA1, CA2, and CA3 use the initials of cornu Ammonis, an earlier name of the hippocampus.
The supramammillary nucleus (SuM), or supramammillary area, is a thin layer of cells in the brain that lies above the mammillary bodies. It can be considered part of the hypothalamus and diencephalon. The nucleus can be divided into medial and lateral sections. The medial SuM, or SuMM, is made of smaller cells which release dopamine and give input to the lateral septal nucleus. The lateral SuM, or SuML, is made of larger cells that project to the hippocampus.
Christine Denny is an American neuroscientist and Associate Professor of Clinical Neurobiology in Psychiatry in the Department of Psychiatry at Columbia University Irving Medical Center in New York City. Denny investigates the molecular mechanisms underlying learning and memory. She developed a novel technique to label neurons that encode specific memories. She used this technique to probe what happens to hippocampal memory traces in different disease states.
References
- 1 2 3 4 5 "Attila Losonczy Biography" (PDF). Columbia University. Retrieved 11 April 2016.
- 1 2 Hogenboom, Melissa (20 February 2014). "Fear suppressing neurons found". BBC News. Retrieved 11 April 2016.
- ↑ Losonczy, Attila. "Central Directions of the Lab". Losonczy Lab. Columbia University. Retrieved 11 April 2016.
- 1 2 Borthwick, Lindsey. "Modeling the Brain on 'Replay': A Q&A with Attila Losonczy". The Kavli Foundation. Retrieved 11 April 2016.
- 1 2 Lovett-Barron, Matthew; Losonczy, Attila (21 February 2014). "Dendritic Inhibition in the Hippocampus Supports Fear Learning". Science. 343 (6173): 857–863. Bibcode:2014Sci...343..857L. doi:10.1126/science.1247485. PMC 4018419 . PMID 24558155.
- ↑ Futurism (15 March 2016). "Scientists Get Their First Glimpse At How New Memories Are Born". Futurism. Retrieved 11 April 2016.
- ↑ Hernandez, Daniela. "The Mysteries of How Newborn Cells May Help Form Memories". Wall Street Journal. Retrieved 11 April 2016.
- 1 2 Underwood, Emily (10 March 2016). "Newborn neurons keep memories crisp and fresh". Science News. Retrieved 11 April 2016.
- ↑ Offord, Catherine (14 March 2016). "Observing Nascent Neurons in Action". The Scientist. Retrieved 11 April 2016.
- 1 2 Hamzelou, Jessica. "Newborn neurons observed in a live brain for first time". New Scientist. Retrieved 11 April 2016.
- ↑ Danielson, Nathan; Kaifosh, Patrick; Lovett-Barron, Matthew; Tsai, Joseph; Denny, Christine; Balough, Elizabeth; Goldberg, Alexander; Drew, Liam; Hen, Rene; Losonczy, Attila; Kheirbek, Mazen (6 April 2016). "Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding". Neuron. 90 (1): 101–112. doi:10.1016/j.neuron.2016.02.019. PMC 4962695 . PMID 26971949.