
Penicillins are a group of antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are chemically synthesised from naturally-produced penicillins. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are members of the β-lactam antibiotics, which are some of the most powerful and successful achievements in modern science. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
Glucono-delta-lactone (GDL), also known as gluconolactone, is a food additive with the E-number E575 used as a sequestrant, an acidifier, or a curing, pickling, or leavening agent. It is a lactone of d-gluconic acid. Pure GDL is a white odorless crystalline powder.
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminate long-stored food and it causes different toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.

The history of penicillin follows a number of observations and discoveries of apparent evidence of antibiotic activity of the mould Penicillium. Following the identification of Penicillium rubens as the source of the compound in 1928 and with the production of pure compound in 1942, penicillin became the first naturally derived antibiotic. There are anecdotes about ancient societies using moulds to treat infections, and in the following centuries many people observed the inhibition of bacterial growth by various moulds. However, it is unknown if the species involved were Penicillium species or if the antimicrobial substances produced were penicillin.
Penicillium aurantiogriseum is a plant pathogen infecting asparagus and strawberry. Chemical compounds isolated from Penicillium aurantiogriseum include anicequol and auranthine.
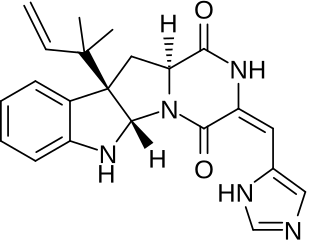
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus Penicillium. It was first isolated from a strain of Penicillium roqueforti, a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.
Leucogenenol is a blood cell stimulating secondary metabolite isolated from the mold Penicillium gilmanii. Its chemical structure was reported; however, later studies determined that the original structure is incorrect and the true chemical structure of leucogenenol remains unknown.
Throughout human history, fungi have been utilized as a source of food and harnessed to ferment and preserve foods and beverages. In the 20th century, humans have learned to harness fungi to protect human health, while industry has utilized fungi for large scale production of enzymes, acids, and biosurfactants. With the advent of modern nanotechnology in the 1980s, fungi have remained important by providing a greener alternative to chemically synthesized nanoparticle.

Aurantiomides are quinazoline alkaloids isolated from the fungus Penicillium aurantiogriseum.

Dideoxyverticillin A, also known as (+)-11,11’-dideoxyverticillin A, is a complex epipolythiodioxopiperazine initially isolated from the marine fungus Penicillium sp. in 1999. It has also been found in the marine fungus Bionectriaceae, and belongs to a class of naturally occurring 2,5-diketopiperazines.
Medicinal fungi are fungi which contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or are under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
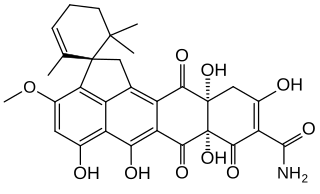
Viridicatumtoxin B is a fungus-derived tetracycline-like antibiotic discovered in 2008. It was isolated from small amounts of penicillium fungi. A synthetic structure matching that of natural viridicatumtoxin B makes possible synthetic variants that match or surpass its antibiotic potency.
Penicillium citrinum is an anamorph, mesophilic fungus species of the genus of Penicillium which produces tanzawaic acid A-D, ACC, Mevastatin, Quinocitrinine A, Quinocitrinine B, and nephrotoxic citrinin. Penicillium citrinum is often found on moldy citrus fruits and occasionally it occurs in tropical spices and cereals. This Penicillium species also causes mortality for the mosquito Culex quinquefasciatus. Because of its mesophilic character, Penicillium citrinum occurs worldwide. The first statin (Mevastatin) was 1970 isolated from this species.
Penicillium decumbens is an anamorph species of the genus of Penicillium which occurs widespread in nature, mainly in subtropical and tropical soil but it also occur in food. Analysis have shown that Penicillium decumbens has antibiotic activity Penicillium decumbens produces the cyclopentenone cyclopenicillone
Penicillium multicolor is an anamorph species of the genus Penicillium which produces alpha-L-fucosidase, tilactase, sclerotiorin, 8-O-Methylsclerotiorinamine, multicolosic acid and isochromophilones.
Penicillium paxilli is an anamorph, saprophytic species of the genus Penicillium which produces paxilline, paxisterol, penicillone, pyrenocine A, paspaline B and verruculogene. Penicillium paxilli is used as a model to study the biochemistry of the indol-diterepene biosysnthesis
Penicillium raistrickii is an anamorph species of fungus in the genus Penicillium which produces griseofulvin, patulin and verruculogen.
Rugulosin is an anthraquinoid mycotoxin with the molecular formula C30H22O10 which is produced by Penicillium species. Rugulosin is hepatotoxic and is cancerogenic.







