
Buprenorphine, sold under the brand name Subutex among others, is an opioid used to treat opioid use disorder, acute pain, and chronic pain. It can be used under the tongue (sublingual), in the cheek (buccal), by injection, as a skin patch (transdermal), or as an implant. For opioid use disorder, the patient must have moderate opioid withdrawal symptoms before buprenorphine can be administered under direct observation of a health-care provider.

An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.

Nociceptin/orphanin FQ (N/OFQ), a 17-amino acid neuropeptide, is the endogenous ligand for the nociceptin receptor. Nociceptin acts as a potent anti-analgesic, effectively counteracting the effect of pain-relievers; its activation is associated with brain functions such as pain sensation and fear learning.

Norbuprenorphine is a major active metabolite of the opioid modulator buprenorphine. It is a μ-opioid, δ-opioid, and nociceptin receptor full agonist, and a κ-opioid receptor partial agonist. In rats, unlike buprenorphine, norbuprenorphine produces marked respiratory depression but with very little antinociceptive effect. In explanation of these properties, norbuprenorphine has been found to be a high affinity P-glycoprotein substrate, and in accordance, shows very limited blood-brain-barrier penetration.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.
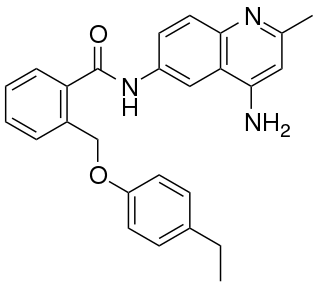
JTC-801 is an opioid analgesic drug used in scientific research.

BU-48 is a drug that is used in scientific research. It is from the oripavine family, related to better-known drugs such as etorphine and buprenorphine.

J-113,397 is an opioid drug which was the first compound found to be a highly selective antagonist for the nociceptin receptor, also known as the ORL-1 receptor. It is several hundred times selective for the ORL-1 receptor over other opioid receptors, and its effects in animals include preventing the development of tolerance to morphine, the prevention of hyperalgesia induced by intracerebroventricular administration of nociceptin, as well as the stimulation of dopamine release in the striatum, which increases the rewarding effects of cocaine, but may have clinical application in the treatment of Parkinson's disease.

Ro64-6198 is an opioid drug used in scientific research. It acts as a potent and selective agonist for the nociceptin receptor, also known as the ORL-1 receptor, with over 100x selectivity over the other opioid receptors. It produces anxiolytic effects in animal studies equivalent to those of benzodiazepine drugs, but has no anticonvulsant effects and does not produce any overt effects on behaviour. However it does impair short-term memory, and counteracts stress-induced anorexia. It also has antitussive effects, and reduces the rewarding and analgesic effects of morphine, although it did not prevent the development of dependence. It has been shown to reduce alcohol self-administration in animals and suppressed relapses in animal models of alcoholism, and ORL-1 agonists may have application in the treatment of alcoholism.
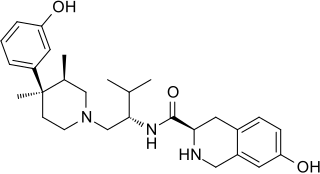
JDTic is a selective, long-acting ("inactivating") antagonist of the κ-opioid receptor (KOR). JDTic is a 4-phenylpiperidine derivative, distantly related structurally to analgesics such as pethidine and ketobemidone, and more closely to the MOR antagonist alvimopan. In addition, it is structurally distinct from other KOR antagonists such as norbinaltorphimine. JDTic has been used to create crystal structures of KOR [ PDB: 4DJH, 6VI4].

Clocinnamox is a selective and irreversible antagonist of the μ-opioid receptor. Closely related compounds include methocinnamox (MCAM) and methoclocinnamox (MCCAM). They were derived via structural modification of buprenorphine. Clocinnamox was first described in the scientific literature by 1992.

Buprenorphine/samidorphan is a combination formulation of buprenorphine and samidorphan which is under development as an add on to antidepressants in treatment-resistant depression (TRD).

Norbuprenorphine-3-glucuronide (N3G) is a major active metabolite of the opioid modulator buprenorphine. It has affinity for the κ-opioid receptor and the nociceptin receptor, but not for the μ- or δ-opioid receptors. Whether N3G acts as an agonist or antagonist of each of the former two respective sites has yet to be determined. In animals, N3G has been found to produce sedation, decreased locomotion, and a small amount of antinociception, properties which are consistent with the effects of κ-opioid receptor agonists. In addition, N3G has been found to reduce tidal volume but not respiratory rate. Unlike norbuprenorphine, but similarly to buprenorphine and buprenorphine-3-glucuronide, N3G is not a substrate for P-glycoprotein. However, due to its highly hydrophilic nature, N3G nonetheless passes the blood-brain-barrier in only very small amounts.

Cebranopadol is an opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.

AT-076 is a so-called opioid "pan" antagonist and is the first reasonably balanced antagonist known of all four opioid receptor types. It acts as a silent antagonist of all four of the opioid receptors, behaving as a competitive antagonist of the μ-opioid receptor and δ-opioid receptor and as a noncompetitive antagonist of the κ-opioid receptor and nociceptin receptor. AT-076 was derived from the selective κ-opioid receptor antagonist JDTic via removal of the 3,4-dimethyl group of the trans-(3R,4R)-dimethyl-4-(3-hydroxyphenyl)piperidine antagonist scaffold which increased affinity for the nociceptin receptor by 10-fold and for the μ- and δ-opioid receptors by 3-6-fold.

Thienorphine is a very potent, extremely long-acting, orally-active opioid analgesic with mixed agonist–antagonist properties which was developed by the Beijing Institute of Pharmacology and Toxicology as a potential treatment for opioid dependence. It is a high-affinity, balanced ligand of the μ-, δ-, and κ-opioid receptors, behaving as a partial agonist of the μ- and κ-opioid receptors and as an antagonist of the δ-opioid receptor. It also possesses relatively low affinity for the nociceptin receptor, where it acts as an antagonist.

AT-121 is an experimental analgesic. It was designed to be bifunctional, acting as an agonist at both the μ-opioid receptor and the nociceptin receptor. The interaction with the nociceptin receptor is expected to block the abuse and dependence-related side effects that are typical of opioids. A study in nonhuman primates found that AT-121 has morphine-like analgesic effects, but suppressed the addictive effects.
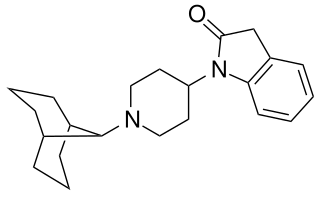
SR-16435 is a drug which acts as a potent partial agonist at both the μ-opioid receptor and nociceptin receptor. In animal studies it was found to be a potent analgesic, with results suggestive of reduced development of tolerance and increased activity against neuropathic pain compared to classic μ-selective agonists.

Ro65-6570 is an opioid drug. It has a potential use in preventing the addiction to other opioids.

SCH-221510 is an experimental opioid drug. It has potential as an analgesic and as a treatment to addiction of certain drugs.




















