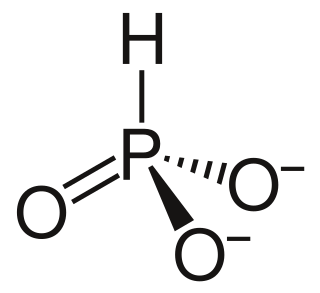
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.

Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.
In industrial chemistry, a stabilizer or stabiliser is a chemical that is used to prevent degradation. Above all, heat and light stabilizers are added to plastic and rubber materials because they ensure safe processing and protect products against aging and weathering. In particular polyvinyl chloride would not be possible without stabilizers.

In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
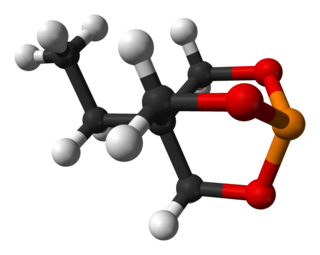
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a phosphite ester used as a ligand in organometallic chemistry. Trimethylolpropane phosphite is sometimes abbreviated to EtCage. It is a white solid that is soluble in organic solvents. It is also highly toxic.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

Triethyl phosphite is an organophosphorus compound, specifically a phosphite ester, with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis.
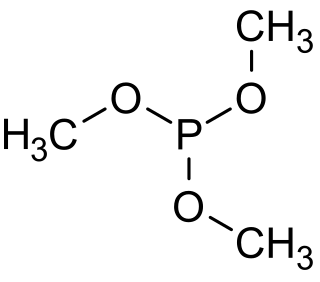
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
Polymer stabilizers are chemical additives which may be added to polymeric materials to inhibit or retard their degradation. Mainly they protect plastic and rubber products against heat, oxidation, and UV light. The biggest quantity of stabilizers is used for polyvinyl chloride (PVC), as the production and processing of this type of plastic would not be possible without stablizing chemicals. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.
Mixed-anion compounds, heteroanionic materials or mixed-anion materials are chemical compounds containing cations and more than one kind of anion. The compounds contain a single phase, rather than just a mixture.
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.
A phosphate phosphite is a chemical compound or salt that contains phosphate and phosphite anions (PO33- and PO43-). These are mixed anion compounds or mixed valence compounds. Some have third anions.
The oxalate phosphites are chemical compounds containing oxalate and phosphite anions. They are also called oxalatophosphites or phosphite oxalates. Oxalate phosphates can form metal organic framework compounds.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.













