Trinitrotoluene, more commonly known as TNT (and more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene), is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.
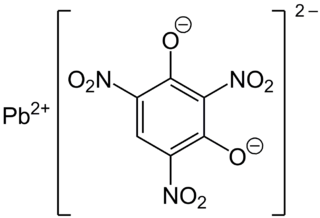
Lead styphnate (lead 2,4,6-trinitroresorcinate, C6HN3O8Pb ), whose name is derived from styphnic acid, is an explosive used as a component in primer and detonator mixtures for less sensitive secondary explosives. Lead styphnate is only slightly soluble in water and methanol. Samples of lead styphnate vary in color from yellow to gold, orange, reddish-brown, to brown. Lead styphnate is known in various polymorphs, hydrates, and basic salts. Normal lead styphnate monohydrate, monobasic lead styphnate, tribasic lead styphnate dihydrate, and pentabasic lead styphnate dehydrate as well as α, β polymorphs of lead styphnate exist.

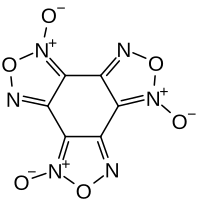
Hexanitrobenzene, also known as HNB, is a nitrobenzene compound in which six nitro groups are bonded to all six positions of a central benzene ring. It is a high-density explosive compound with chemical formula C6N6O12, obtained by oxidizing the amine group of pentanitroaniline with hydrogen peroxide in sulfuric acid.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Silver perchlorate is the chemical compound with the formula AgClO4. This white solid forms a monohydrate and is mildly deliquescent. It is a useful source of the Ag+ ion, although the presence of perchlorate presents risks. It is used as a catalyst in organic chemistry.
The Staudinger reaction is a chemical reaction of an organic azide with a phosphine or phosphite produces an iminophosphorane. The reaction was discovered by and named after Hermann Staudinger. The reaction follows this stoichiometry:

TATB, triaminotrinitrobenzene or 2,4,6-triamino-1,3,5-trinitrobenzene is an aromatic explosive, based on the basic six-carbon benzene ring structure with three nitro functional groups (NO2) and three amine (NH2) groups attached, alternating around the ring.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its detonation velocity is 7,350 meters per second, which is comparable to TATB (triaminotrinitrobenzene).

2,4,6-Tris(trinitromethyl)-1,3,5-triazine is a chemical compound that is a derivative of triazine first prepared in 1995. It is synthesized by destructive nitration of 2,4,6-tricarboxyl-1,3,5-triazine. It is noteworthy for having more nitro groups than it does carbon atoms, thus potentially being useful as an oxygen source, or added to oxygen-poor explosives to increase their power.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.

Group 2 organometallic chemistry refers to the organic derivativess of any group 2 element. It is a subtheme to main group organometallic chemistry. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are typically limited to academic interests.
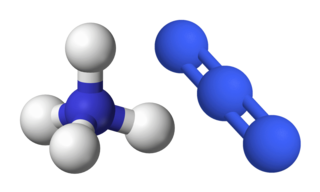
Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. Like other inorganic azides, this colourless crystalline salt is a powerful explosive, although it has a remarkably low sensitivity. [NH4]N3 is physiologically active and inhalation of small amounts causes headaches and palpitations. It was first obtained by Theodor Curtius in 1890, along with other azides.

Silicon tetraazide is a thermally unstable binary compound of silicon and nitrogen with a nitrogen content of 85.7%. This high-energy compound combusts spontaneously and can only be studied in a solution. A further coordination to a six-fold coordinated structure such as a hexaazidosilicate ion [Si(N3)6]2− or as an adduct with bidentate ligands Si(N3)4·L2 will result in relatively stable, crystalline solids that can be handled at room temperature.

Cyanuric triazide (C3N12 or (NCN3)3) is described as an environmentally friendly, low toxicity, and organic primary explosive with a detonation velocity of about 7,300 m s−1 and a autoignition temperature of 205 °C.
Hydrazinium azide or hydrazine azide is a chemical compound with formula H
5N
5 or [N
2H+
5][N−
3]. It is a salt of the hydrazinium cation N
2H+
5 and the azide anion N−
3. It can be seen as a derivative of hydrazine N
2H
4 and hydrazoic acid HN
3. It is an unstable solid.

(Benzene)ruthenium dichloride dimer is the organoruthenium compound with the formula [(C6H6)RuCl2]2. This red-coloured, diamagnetic solid is a reagent in organometallic chemistry and homogeneous catalysis.

Boron triazide, also known as triazidoborane, is a thermally unstable compound of boron and nitrogen with a nitrogen content of 92.1 %. Formally, it is the triazido derivative of borane and is a covalent inorganic azide. The high-energy compound, which has the propensity to undergo spontaneous explosive decomposition, was first described in 1954 by Egon Wiberg and Horst Michaud of the University of Munich.