
Elias James Corey is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Biliary atresia, also known as extrahepatic ductopenia and progressive obliterative cholangiopathy, is a childhood disease of the liver in which one or more bile ducts are abnormally narrow, blocked, or absent. It can be congenital or acquired. It has an incidence of one in 10,000–15,000 live births in the United States, and a prevalence of one in 16,700 in the British Isles. Biliary atresia is most common in East Asia, with a frequency of one in 5,000.

Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.

Cisplatin is a chemical compound with formula cis-[Pt(NH3)2Cl2]. It is a coordination complex of platinum that is used as a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, brain tumors and neuroblastoma. It is given by injection into a vein.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.
Click chemistry is an approach to chemical synthesis that emphasizes efficiency, simplicity, selectivity, and modularity in chemical processes used to join molecular building blocks. It includes both the development and use of "click reactions", a set of simple, biocompatible chemical reactions that meet specific criteria like high yield, fast reaction rates, and minimal byproducts. It was first fully described by K. Barry Sharpless, Hartmuth C. Kolb, and M. G. Finn of The Scripps Research Institute in 2001. In this seminal paper, Sharpless argued that synthetic chemistry could emulate the way nature constructs complex molecules, using efficient reactions to join together simple, non-toxic building blocks.
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.

N-Nitrosonornicotine (NNN) is a tobacco-specific nitrosamine produced during the curing and processing of tobacco.
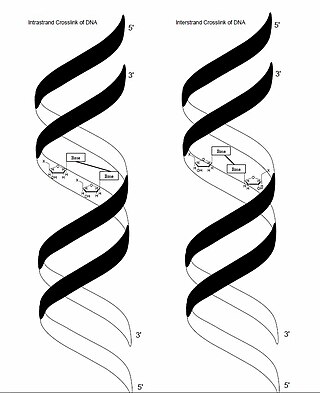
In genetics, crosslinking of DNA occurs when various exogenous or endogenous agents react with two nucleotides of DNA, forming a covalent linkage between them. This crosslink can occur within the same strand (intrastrand) or between opposite strands of double-stranded DNA (interstrand). These adducts interfere with cellular metabolism, such as DNA replication and transcription, triggering cell death. These crosslinks can, however, be repaired through excision or recombination pathways.
Isoflavonoids are a class of flavonoid phenolic compounds, many of which are biologically active. Isoflavonoids and their derivatives are sometimes referred to as phytoestrogens, as many isoflavonoid compounds have biological effects via the estrogen receptor.

Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule. Methods to conjugate biomolecules are applied in various field, including medicine, diagnostics, biocatalysis and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme function, determining protein biodistribution, imaging specific biomarkers, and delivering drugs to targeted cells.

Monoamine oxidase B (MAO-B) is an enzyme that in humans is encoded by the MAOB gene.
At its simplest, the adductome is the totality of chemical adducts that are present in particular cellular macromolecules such as DNA, and RNA, or proteins found within the organism. These adducts can detrimentally alter the chemical properties of these macromolecules and are therefore also referred to as damage. Adducts may arise as a consequence of the chemical reaction between a given "physicochemical agent to which an organism is exposed across the lifespan". These physicochemical agents can be exogenous in origin, and include ionizing and non-ionizing radiation, the diet, lifestyle factors, pollution, and xenobiotics. They made damage the macromolecules directly, or indirectly e.g., some xenobiotic substances require metabolism of the xenobiotic to produce a chemically reactive metabolite which can then form a covalent bond with the endogenous macromolecule. Agents that damage macromolecules can also arise from endogenous sources, such as reactive oxygen species that are a side product of normal respiration, leading to the formation of oxidatively damaged DNA etc., or other reactive species e.g., reactive nitrogen, sulphur, carbon, selenium and halogen species.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

Phosphasilenes or silylidenephosphanes are a class of compounds with silicon-phosphorus double bonds. Since the electronegativity of phosphorus (2.1) is higher than that of silicon (1.9), the "Si=P" moiety of phosphasilene is polarized. The degree of polarization can be tuned by altering the coordination numbers of the Si and P centers, or by modifying the electronic properties of the substituents. The phosphasilene Si=P double bond is highly reactive, yet with the choice of proper substituents, it can be stabilized via donor-acceptor interaction or by steric congestion.
An Artificial Metalloenzyme (ArM) is a designer metalloprotein, not found in nature, which can catalyze desired chemical reactions. Despite fitting into classical enzyme categories, ArMs also have potential in new-to-nature chemical reactivity like catalysing Suzuki coupling, Metathesis etc., which were never reported among natural enzymatic reactions.

Stephen L. Craig is the William T. Miller Professor of Chemistry at Duke University. He is the director of the Center for Molecularly Optimized Networks, a NSF Center for Chemical Innovation.
In organic chemistry, carboboration describes an addition of both a carbon and a boron moiety to certain carbon-containing double and triple bonds, such as alkenes, alkynes, and allenes.














