
An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

Photosynthesis is a biological process used by many cellular organisms to convert light energy into chemical energy, which is stored in organic compounds that can later be metabolized through cellular respiration to fuel the organism's activities. The term usually refers to oxygenic photosynthesis, where oxygen is produced as a byproduct and some of the chemical energy produced is stored in carbohydrate molecules such as sugars, starch, glycogen and cellulose, which are synthesized from endergonic reaction of carbon dioxide with water. Most plants, algae and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the biological energy necessary for complex life on Earth.

Redox is a type of chemical reaction in which the oxidation states of a substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
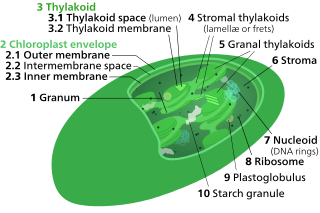
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stromal thylakoids, which join granum stacks together as a single functional compartment.

Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae, and cyanobacteria. These membranes are located inside the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: PSI and PSII.

Photosystem I is one of two photosystems in the photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane protein complex that uses light energy to catalyze the transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin. Ultimately, the electrons that are transferred by Photosystem I are used to produce the moderate-energy hydrogen carrier NADPH. The photon energy absorbed by Photosystem I also produces a proton-motive force that is used to generate ATP. PSI is composed of more than 110 cofactors, significantly more than Photosystem II.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel. Photocatalytic water splitting converts water into hydrogen and oxygen and is a major research topic of artificial photosynthesis. Light-driven carbon dioxide reduction is another process studied that replicates natural carbon fixation.

A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and pheophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The free energy created is then used, via a chain of nearby electron acceptors, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide towards carbon dioxide, eventually producing glucose. These electron transfer steps ultimately result in the conversion of the energy of photons to chemical energy.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system also known as micro fuel cell that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds on the anode to oxidized compounds such as oxygen on the cathode through an external electrical circuit. MFCs produce electricity by using the electrons derived from biochemical reactions catalyzed by bacteria.Comprehensive Biotechnology MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFCs have started to find commercial use in wastewater treatment.

Biohydrogen is H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic microorganisms are capable to produce H2 directly from water splitting using light as energy source.
An enzymatic biofuel cell is a specific type of fuel cell that uses enzymes as a catalyst to oxidize its fuel, rather than precious metals. Enzymatic biofuel cells, while currently confined to research facilities, are widely prized for the promise they hold in terms of their relatively inexpensive components and fuels, as well as a potential power source for bionic implants.
Electromethanogenesis is a form of electrofuel production where methane is produced by direct biological conversion of electrical current and carbon dioxide.

Light-dependent reactions refers to certain photochemical reactions that are involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions, the first occurs at photosystem II (PSII) and the second occurs at photosystem I (PSI).
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the German electrochemist Heinz Gerischer. The interest in this domain is high in the context of development of renewable energy conversion and storage technology.
A Bioelectrochemical reactor is a type of bioreactor where bioelectrochemical processes are used to degrade/produce organic materials using microorganisms. This bioreactor has two compartments: The anode, where the oxidation reaction takes place; And the cathode, where the reduction occurs. At these sites, electrons are passed to and from microbes to power reduction of protons, breakdown of organic waste, or other desired processes. They are used in microbial electrosynthesis, environmental remediation, and electrochemical energy conversion. Examples of bioelectrochemical reactors include microbial electrolysis cells, microbial fuel cells, enzymatic biofuel cells, electrolysis cells, microbial electrosynthesis cells, and biobatteries.
Geopsychrobacter electrodiphilus is a species of bacteria, the type species of its genus. It is a psychrotolerant member of its family, capable of attaching to the anodes of sediment fuel cells and harvesting electricity by oxidation of organic compounds to carbon dioxide and transferring the electrons to the anode.
Arsenate-reducing bacteria are bacteria which reduce arsenates. Arsenate-reducing bacteria are ubiquitous in arsenic-contaminated groundwater (aqueous environment). Arsenates are salts or esters of arsenic acid (H3AsO4), consisting of the ion AsO43−. They are moderate oxidizers that can be reduced to arsenites and to arsine. Arsenate can serve as a respiratory electron acceptor for oxidation of organic substrates and H2S or H2. Arsenates occur naturally in minerals such as adamite, alarsite, legrandite, and erythrite, and as hydrated or anhydrous arsenates. Arsenates are similar to phosphates since arsenic (As) and phosphorus (P) occur in group 15 (or VA) of the periodic table. Unlike phosphates, arsenates are not readily lost from minerals due to weathering. They are the predominant form of inorganic arsenic in aqueous aerobic environments. On the other hand, arsenite is more common in anaerobic environments, more mobile, and more toxic than arsenate. Arsenite is 25–60 times more toxic and more mobile than arsenate under most environmental conditions. Arsenate can lead to poisoning, since it can replace inorganic phosphate in the glyceraldehyde-3-phosphate --> 1,3-biphosphoglycerate step of glycolysis, producing 1-arseno-3-phosphoglycerate instead. Although glycolysis continues, 1 ATP molecule is lost. Thus, arsenate is toxic due to its ability to uncouple glycolysis. Arsenate can also inhibit pyruvate conversion into acetyl-CoA, thereby blocking the TCA cycle, resulting in additional loss of ATP.
A microbial desalination cell (MDC) is a biological electrochemical system that implements the use of electro-active bacteria to power desalination of water in situ, resourcing the natural anode and cathode gradient of the electro-active bacteria and thus creating an internal supercapacitor. Available water supply has become a worldwide endemic as only .3% of the earth's water supply is usable for human consumption, while over 99% is sequestered by oceans, glaciers, brackish waters, and biomass. Current applications in electrocoagulation, such as microbial desalination cells, are able to desalinate and sterilize formerly unavailable water to render it suitable for safe water supply. Microbial desalination cells stem from microbial fuel cells, deviating by no longer requiring the use of a mediator and instead relying on the charged components of the internal sludge to power the desalination process. Microbial desalination cells therefore do not require additional bacteria to mediate the catabolism of the substrate during biofilm oxidation on the anodic side of the capacitor. MDCs and other bio-electrical systems are favored over reverse osmosis, nanofiltration and other desalination systems due to lower costs, energy and environmental impacts associated with bio-electrical systems.
Microbial electrochemical technologies (METs) use microorganisms as electrochemical catalyst, merging the microbial metabolism with electrochemical processes for the production of bioelectricity, biofuels, H2 and other valuable chemicals. Microbial fuel cells (MFC) and microbial electrolysis cells (MEC) are prominent examples of METs. While MFC is used to generate electricity from organic matter typically associated with wastewater treatment, MEC use electricity to drive chemical reactions such as the production of H2 or methane. Recently, microbial electrosynthesis cells (MES) have also emerged as a promising MET, where valuable chemicals can be produced in the cathode compartment. Other MET applications include microbial remediation cell, microbial desalination cell, microbial solar cell, microbial chemical cell, etc.,.







