
Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

N-Oxoammonium salts are a class of organic compounds with the formula [R1R2+N=O]X−. The cation [R1R2+N=O] is of interest for the dehydrogenation of alcohols. Oxoammonium salts are diamagnetic, whereas the nitroxide has a doublet ground state. A prominent N-oxoammonium salt is prepared by oxidation of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, commonly referred to as [TEMPO]+. A less expensive analogue is Bobbitt's salt.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
The Balz–Schiemann reaction is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to fluorobenzene and some related derivatives, including 4-fluorobenzoic acid.

Triacetonamine is an organic compound with the formula OC(CH2CMe2)2NH (where Me = CH3). It is a colorless or white solid that melts near room temperature. The compound is an intermediate in the preparation of 2,2,6,6-tetramethylpiperidine, a sterically hindered base and precursor to the reagent called TEMPO. Triacetonamine is formed by the poly-aldol condensation of acetone in the presence of ammonia and calcium chloride:
Selectfluor, a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the nucleophillic base DABCO. It is a colourless salt that tolerates air and even water. It has been commercialized for use for electrophilic fluorination.

Tetrakis(acetonitrile)copper(I) hexafluorophosphate is a salt with the formula [Cu(CH3CN)4]PF6. It is a colourless solid that is used in the synthesis of other copper complexes. The cation [Cu(CH3CN)4]+ is a well-known example of a transition metal nitrile complex.

Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid is used in organic synthesis as a nitrosating agent.

Copper(II) tetrafluoroborate is any inorganic compound with the formula Cu(H2O)x(BF4)2. As usually encountered, it is assumed to be the hexahydrate (x = 6), but this salt can be partially dehydrated to the tetrahydrate. Regardless, these compounds are aquo complexes of copper in its +2 oxidation state, with two weakly coordinating tetrafluoroborate anions.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.

Oxoammonium-catalyzed oxidation reactions involve the conversion of organic substrates to more highly oxidized materials through the action of an N-oxoammonium species. Nitroxides may also be used in catalytic amounts in the presence of a stoichiometric amount of a terminal oxidant. Nitroxide radical species used are either 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or derivatives thereof.

Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]−, indicating the presence of fluorinated aryl (ArF) groups. It is sometimes referred to as Kobayashi's anion in honour of Hiroshi Kobayashi who led the team that first synthesised it. More commonly it is affectionately nicknamed "BARF." The BARF ion is also abbreviated BArF24−, to distinguish it from the closely related BArF−
20, [(C6F5)4B]−. However, for a small group of chemists, the anion is abbreviated as TFPB otherwise, short for Tetrakis[3,5-bis(triFluoromethyl)Phenyl]Borate.

(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl, commonly known as TEMPO, is a chemical compound with the formula (CH2)3(CMe2)2NO. This heterocyclic compound is a red-orange, sublimable solid. As a stable aminoxyl radical, it has applications in chemistry and biochemistry. TEMPO is used as a radical marker, as a structural probe for biological systems in conjunction with electron spin resonance spectroscopy, as a reagent in organic synthesis, and as a mediator in controlled radical polymerization.
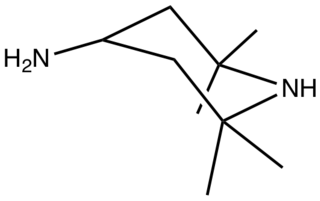
4-Amino-2,2,6,6-tetramethyl-4-piperidine is an organic compound with the formula H2NCH(CH2CMe2)2NH (where Me = CH3). Classified as a diamine, it is a colorless oily liquid.

James McCue Bobbitt was an American chemist and academic who taught chemistry at the University of Connecticut from 1956 to 1991 and developed the Bobbitt reaction.

Ammonium tetrafluoroborate (or ammonium fluoroborate) is an inorganic salt composed of the ammonium cation and the tetrafluoroborate anion, with the chemical formula NH4BF4. When heated to decomposition, ammonium tetrafluoroborate releases toxic fumes of hydrogen fluoride, nitrogen oxides, and ammonia.

The Babler oxidation, also known as the Babler-Dauben oxidation, is an organic reaction for the oxidative transposition of tertiary allylic alcohols to enones using pyridinium chlorochromate (PCC):
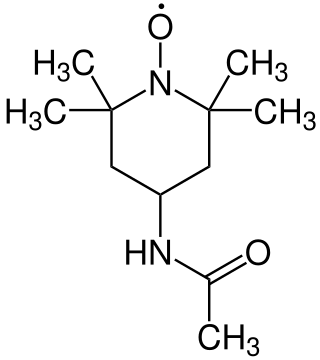
4-acetamido-TEMPO is a stable radical used for oxidation reactions in organic chemistry. It is a derivative of TEMPO, from which it differs by the additional acetamide group.

















