
Staphylococcus epidermidis is a Gram-positive bacterium, and one of over 40 species belonging to the genus Staphylococcus. It is part of the normal human microbiota, typically the skin microbiota, and less commonly the mucosal microbiota and also found in marine sponges. It is a facultative anaerobic bacteria. Although S. epidermidis is not usually pathogenic, patients with compromised immune systems are at risk of developing infection. These infections are generally hospital-acquired. S. epidermidis is a particular concern for people with catheters or other surgical implants because it is known to form biofilms that grow on these devices. Being part of the normal skin microbiota, S. epidermidis is a frequent contaminant of specimens sent to the diagnostic laboratory.
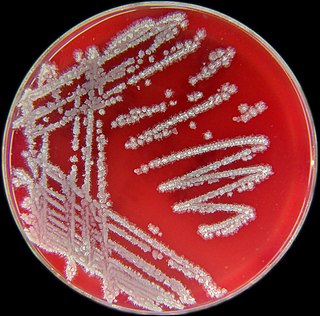
Bacillus licheniformis is a bacterium commonly found in the soil. It is found on bird feathers, especially chest and back plumage, and most often in ground-dwelling birds and aquatic species.
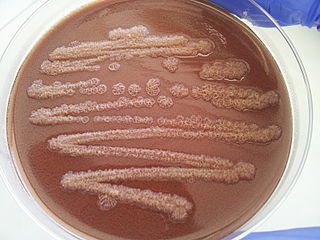
Pseudomonas stutzeri is a Gram-negative soil bacterium that is motile, has a single polar flagellum, and is classified as bacillus, or rod-shaped. While this bacterium was first isolated from human spinal fluid, it has since been found in many different environments due to its various characteristics and metabolic capabilities. P. stutzeri is an opportunistic pathogen in clinical settings, although infections are rare. Based on 16S rRNA analysis, this bacterium has been placed in the P. stutzeri group, to which it lends its name.
Methylobacterium mesophilicum is a Gram-negative, soil-dwelling bacterium, reported to be an opportunistic pathogen in immunocomprimised patients.
Klebsiella aerogenes, previously known as Enterobacter aerogenes, is a Gram-negative, oxidase-negative, catalase-positive, citrate-positive, indole-negative, rod-shaped bacterium. Capable of motility via peritrichous flagella, the bacterium is approximately 1–3 microns in length.
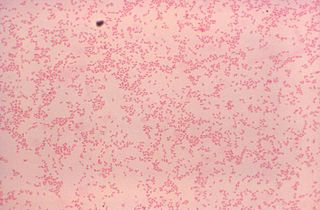
Brucella canis is a Gram-negative bacterium in the family Brucellaceae that causes brucellosis in dogs and other canids. It is a non-motile short-rod or coccus-shaped organism, and is oxidase, catalase, and urease positive. B. canis causes infertility in both male and female dogs. It can also cause inflammation in the eyes. The hosts of B. canis ranges from domestic animals to foxes and coyotes. It is passed from species to species via genital fluids. Treatments such as spaying, neutering, and long-term antibiotics have been used to combat B. canis. The species was first described in the United States in 1966 where mass abortions of beagles were documented. Brucella canis can be found in both pets and wild animals and lasts the lifespan of the animal it has affected. B. canis has two distinct circular chromosomes that can attribute to horizontal gene transfer.
Vibrio furnissii is a Gram-negative, rod-shaped bacterium. Its type strain is ATCC 35016. V. furnissii is aerogenic (gas-producing), and uses L-rhamnose, L-arginine, L-arabinose, maltose, and D-mannitol, but not L-lysine, L-ornithine, or lactose. It has been isolated from patients with gastroenteritis, bacteremia, skin lesions, and sepsis.
Brucella anthropi is a bacterium. Before 2020 it was listed as Ochrobactrum anthropi. The type strain is strain CIP 82.115. B. anthropi strains are rod-shaped, aerobic, gram-negative, non-pigmented and motile by means of peritrichous flagella. One strain is able to break down Piracetam.
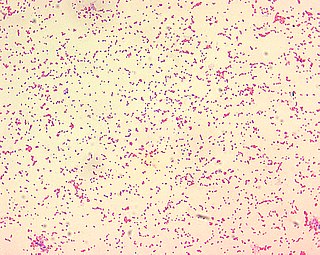
Brucella ceti is a gram negative bacterial pathogen of the Brucellaceae family that causes brucellosis in cetaceans. Brucella ceti has been found in both classes of cetaceans, mysticetes and odontocetes. Brucellosis in some dolphins and porpoises can result in serious clinical signs including fetal abortions, male infertility, neurobrucellosis, cardiopathies, bone and skin lesions, stranding events, and death.
Laribacter hongkongensis is a species of bacteria. It is facultatively anaerobic, non-sporulating, gram-negative, seagull- or spiral rod-shaped. It is a potential human pathogen. Laribacter hongkongensis has been isolated from human cases of diarrhea, but its role in causing diarrhea is unproven, even though it has been hypothesized. Additional studies are needed to better define its role as a possible enteric pathogen. These should include: case control studies designed to differentiate infection from colonization-transient passage, fulfilling Koch's postulates and Bradford-Hill's criteria on association vs. causation, possible virulence factors, animal models, host factors, antibody responses based on serodiagnostic testing, and human volunteer studies. The lessons learned from trying to establish the etiological role of the bacteria genera Aeromonas, Plesiomonas, and Edwardsiella in human diarrhea seem especially applicable for Laribacter. All four genera are isolated from extraintestinal infections, are apparently found in the aquatic environment, and epidemiological associations include eating fish and foreign travel. Even after over 50 years’ experience with the former three genera their etiological role in an individual case of human diarrhea is difficult to determine without extensive studies. For all four of these genera the critical issue will be differentiating infection from colonization or transient passage in the intestine.
Brucella microti is a species of bacteria first isolated from the common vole, Microtus arvalis. Its genome has been sequenced. It is Gram-negative, non-motile, non-spore-forming, and coccoid, with the type strain CCM 4915T. It is pathogenic.
Brucella haematophila is a gram-negative, oxidase-positive, non-spore-forming, non-motile bacteria from the genus of Brucella which was isolated from a man in Falun in Sweden.
Brucella intermedia is a bacterium from the genus of Brucella. It was first described by Velasco and others in 1998. It causes diseases in humans only rarely, with single case reports of cholangitis following liver transplantation, bacteremia in a patient with bladder cancer, a pelvic abscess after abdominal surgery, dyspepsia, endophthalmitis in the presence of a foreign body, pneumonia, and endocarditis.
Brucella pecoris is a gram-negative, oxidase-positive, non-spore-forming, rod-shaped non-motile bacteria from the genus of Brucella which was isolated from genitourinary lymph node of a sheep in Bosnia and Herzegovina.
Brucella pituitosa is a gram-negative, oxidase-positive and catalase-positive, non-spore-forming, non-motile bacteria from the genus of Brucella which was isolated from industrial environment in Sweden.
Brucella pseudogrignonensis is a gram-negative, oxidase-positive, non-spore-forming, non-motile bacteria from the genus of Brucella which was isolated from blood of a man in Göteborg in Sweden.
Brucella rhizosphaerae is a gram-negative, oxidase-positive bacteria from the genus of Brucella which was isolated from rhizosphere from a potato in Austria.
Brucella thiophenivorans is a gram-negative, oxidase-positive non-spore-forming non-motile bacteria from the genus of Brucella which was isolated from waste water in Germany.

Corynebacterium xerosis is a Gram-positive, rod-shaped bacterium in the genus Corynebacterium. Although it is frequently a harmless commensal organism living on the skin and mucus membranes, C. xerosis is also a clinically-relevant opportunistic pathogen that has been attributed to a number of different infections in animals and humans. However, its actual prominence in human medicine is up for debate due to early difficulties distinguishing it from other Corynebacterium species in clinical isolates.
Janet Gilsdorf is an American pediatric infectious diseases physician, scientist, and writer at the University of Michigan. Her research has focused on the pathogenic, molecular, and epidemiologic features of the bacterium Haemophilus influenzae. She served as the Director of the Division of Pediatric Infectious Diseases in the University of Michigan Health System from 1989 to 2012 and Co-Director of the Center for Molecular and Clinical Epidemiology of Infectious Diseases at the School of Public Health at the University of Michigan from 2000 – 2015. In addition to her scientific publications, she is also the author of two novels, one memoir, one non-fiction book, and a number of medically-oriented essays.





