
Pharmacognosy is the study of crude drugs obtained from medicinal plants, animals, fungi, and other natural sources. The American Society of Pharmacognosy defines pharmacognosy as "the study of the physical, chemical, biochemical, and biological properties of drugs, drug substances, or potential drugs or drug substances of natural origin as well as the search for new drugs from natural sources".

Medicinal plants, also called medicinal herbs, have been discovered and used in traditional medicine practices since prehistoric times. Plants synthesize hundreds of chemical compounds for various functions, including defense and protection against insects, fungi, diseases, and herbivorous mammals.

Tigecycline, sold under the brand name Tygacil, is an tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.

Melphalan, sold under the brand name Alkeran among others, is a chemotherapy medication used to treat multiple myeloma, ovarian cancer, melanoma, and AL amyloidosis. It is taken by mouth or by injection into a vein.

Norelgestromin, or norelgestromine, sold under the brand names Evra and Ortho Evra among others, is a progestin medication which is used as a method of birth control for women. The medication is available in combination with an estrogen and is not available alone. It is used as a patch that is applied to the skin.

Podophyllotoxin (PPT) is the active ingredient in Podofilox, which is a medical cream that is used to treat genital warts and molluscum contagiosum. It is not recommended in HPV infections without external warts. It can be applied either by a healthcare provider or the person themselves.
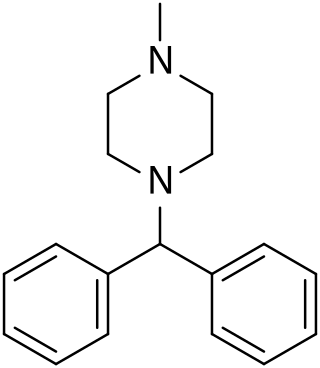
Cyclizine, sold under a number of brand names, is a medication used to treat and prevent nausea, vomiting and dizziness due to motion sickness or vertigo. It may also be used for nausea after general anaesthesia or that which developed from opioid use. It is taken by mouth, in the rectum, or injected into a vein.
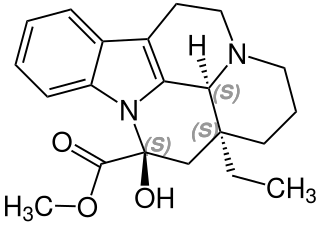
Vincamine is a monoterpenoid indole alkaloid found in the leaves of Vinca minor, comprising about 25-65% of its indole alkaloids by weight. It can also be synthesized from related alkaloids.

Etynodiol diacetate, or ethynodiol diacetate, sold under the brand names Demulen and Femulen among others, is a progestin medication which is used in birth control pills. The medication is available only in combination with an estrogen. It is taken by mouth.

Fospropofol (INN), often used as the disodium salt is an intravenous sedative-hypnotic agent. It is currently approved for use in sedation of adult patients undergoing diagnostic or therapeutic procedures such as endoscopy.

JWH-200 (WIN 55,225) is an analgesic chemical from the aminoalkylindole family that acts as a cannabinoid receptor agonist. Its binding affinity, Ki at the CB1 receptor is 42 nM, around the same as that of THC, but its analgesic potency in vivo was higher than that of other analogues with stronger CB1 binding affinity in vitro, around 3 times that of THC but with less sedative effect, most likely reflecting favourable pharmacokinetic characteristics. It was discovered in 1991 by Sterling Drug as a potential analgesic following the earlier identification of related compounds such as pravadoline and WIN 55,212-2.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Tolufazepam is a drug that is a benzodiazepine derivative. Studies have shown tolufazepam to have anticonvulsant and anxiolytic activity in animal subjects, including convulsions elicited by pentylenetetrazol.

Piperoxan, also known as benodaine, was the first antihistamine to be discovered. This compound, derived from benzodioxan, was prepared in the early 1930s by Daniel Bovet and Ernest Fourneau at the Pasteur Institute in France. Formerly investigated by Fourneau as an α-adrenergic-blocking agent, they demonstrated that it also antagonized histamine-induced bronchospasm in guinea pigs, and published their findings in 1933. Bovet went on to win the 1957 Nobel Prize in Physiology or Medicine for his contribution. One of Bovet and Fourneau's students, Anne-Marie Staub, published the first structure–activity relationship (SAR) study of antihistamines in 1939. Piperoxan and analogues themselves were not clinically useful due to the production of toxic effects in humans and were followed by phenbenzamine (Antergan) in the early 1940s, which was the first antihistamine to be marketed for medical use.

Budesonide, sold under the brand name Pulmicort among others, is a medication of the corticosteroid type. It is available as an inhaler, nebulization solution, pill, nasal spray, and rectal forms. The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease (COPD). The nasal spray is used for allergic rhinitis and nasal polyps. The pills in a delayed release form and rectal forms may be used for inflammatory bowel disease including Crohn's disease, ulcerative colitis, and microscopic colitis.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.
Ivan Addae-Mensah, is a Ghanaian chemist and university administrator who served as the Vice-Chancellor of the University of Ghana, Legon from 1996 to 2002. He is an Emeritus Professor of Chemistry at the same institution. He is a Life Fellow of the Royal Society of Chemistry, Fellow of the Ghana Academy of Arts and Sciences and a Fellow of the Ghana Chemical Society.

Becampanel (INN) (code name AMP397) is a quinoxalinedione derivative drug which acts as a competitive antagonist of the AMPA receptor (IC50 = 11 nM). It was investigated as an anticonvulsant for the treatment of epilepsy by Novartis, and was also looked at as a potential treatment for neuropathic pain and cerebral ischemia, but never completed clinical trials.

Oprozomib is an orally active second-generation proteasome inhibitor developed by Proteolix, which was acquired by Onyx Pharmaceuticals, an Amgen subsidiary, in 2009. It selectively inhibits chymotrypsin-like activity of both the constitutive proteasome (PSMB5) and immunoproteasome (LMP7).


















