Polyvinyl chloride is the world's third-most widely produced synthetic polymer of plastic. About 40 million tons of PVC are produced each year.

A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Chloroethane, commonly known as ethyl chloride, is a chemical compound with chemical formula CH3CH2Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor.
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). About 13 million metric tonne are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM. Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills. In the past VCM was used as a refrigerant.

Lamination is the technique/process of manufacturing a material in multiple layers, so that the composite material achieves improved strength, stability, sound insulation, appearance, or other properties from the use of the differing materials, such as plastic. A laminate is a permanently assembled object created using heat, pressure, welding, or adhesives. Various coating machines, machine presses and calendering equipment are used.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).

Vinyl composition tile (VCT) is a finished flooring material used primarily in commercial and institutional applications. Modern vinyl floor tiles and sheet flooring and versions of those products sold since the early 1980s are composed of colored polyvinyl chloride (PVC) chips formed into solid sheets of varying thicknesses by heat and pressure. Floor tiles are cut into modular shapes such 12-by-12-inch squares or 12-by-24-inch rectangles. In installation the floor tiles or sheet flooring are applied to a smooth, leveled sub-floor using a specially formulated vinyl adhesive or tile mastic that remains pliable. In commercial applications some tiles are typically waxed and buffed using special materials and equipment.

Plastic wrap, cling film, Saran wrap, cling wrap, Glad wrap or food wrap is a thin plastic film typically used for sealing food items in containers to keep them fresh over a longer period of time. Plastic wrap, typically sold on rolls in boxes with a cutting edge, clings to many smooth surfaces and can thus remain tight over the opening of a container without adhesive. Common plastic wrap is roughly 0.0005 inches thick. The trend has been to produce thinner plastic wrap, particularly for household use, so now the majority of brands on shelves around the world are 8, 9 or 10 μm thick.

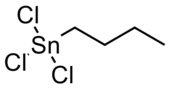
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.
Closed-cell PVC foamboard is a lightweight rigid material used primarily in the manufacture of signs and displays. It is considered robust for outdoor use, being immune to rain and resistant to wind and sunlight.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.

Tributyltin (TBT) is an umbrella term for a class of organotin compounds which contain the (C4H9)3Sn group, with a prominent example being tributyltin oxide. For 40 years TBT was used as a biocide in anti-fouling paint, commonly known as bottom paint, applied to the hulls of oceangoing vessels. Bottom paint improves ship performance and durability as it reduces the rate of biofouling, the growth of organisms on the ship's hull. The TBT slowly leaches out into the marine environment where it is highly toxic toward nontarget organisms. TBT toxicity can lead to biomagnification or bioaccumulation within such nontarget organisms like invertebrates, vertebrates, and a variety of mammals. TBT is also an obesogen. After it led to collapse of local populations of organisms, TBT was banned.

Ruthenium(IV) oxide is the inorganic compound with the formula RuO2. This black solid is the most common oxide of ruthenium. It is widely used as an electrocatalyst for producing chlorine, chlorine oxides, and O2. Like many dioxides, RuO2 adopts the rutile structure.

Dibutyl phthalate (DBP) is an organic compound which is commonly used as a plasticizer because of its low toxicity and wide liquid range. With the chemical formula C6H4(CO2C4H9)2, it is a colorless oil, although commercial samples are often yellow.
Self-cleaning glass is a specific type of glass with a surface that keeps itself free of dirt and grime.

Glass production involves two main methods – the float glass process that produces sheet glass, and glassblowing that produces bottles and other containers. It has been done in a variety of ways during the history of glass.
VinyLoop is a proprietary physical plastic recycling process for polyvinyl chloride (PVC). It is based on dissolution in order to separate PVC from other materials or impurities.

Tantalum(V) ethoxide is a metalorganic compound with formula Ta2(OC2H5)10, often abbreviated as Ta2(OEt)10. It is a colorless solid that dissolves in some organic solvents but hydrolyzes readily. It is used to prepare films of tantalum(V) oxide.