
Clathrin is a protein that plays a role in the formation of coated vesicles. Clathrin was first isolated by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle. The protein's name refers to this lattice structure, deriving from Latin clathri meaning lattice. Barbara Pearse named the protein clathrin at the suggestion of Graeme Mitchison, selecting it from three possible options. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection.
AP180 is a protein that plays an important role in clathrin-mediated endocytosis of synaptic vesicles. It is capable of simultaneously binding both membrane lipids and clathrin and is therefore thought to recruit clathrin to the membrane of newly invaginating vesicles. In Drosophila melanogaster, deletion of the AP180 homologue, leads to enlarged but much fewer vesicles and an overall decrease in transmitter release. In D. melanogaster it was also shown that AP180 is also required for either recycling vesicle proteins and/or maintaining the distribution of both vesicle and synaptic proteins in the nerve terminal. A ubiquitous form of the protein in mammals, CALM, is named after its association with myeloid and lymphoid leukemias where some translocations map to this gene. The C-terminus of AP180 is a powerful and specific inhibitor of clathrin-mediated endocytosis.
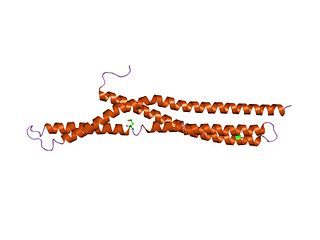
In molecular biology, BAR domains are highly conserved protein dimerisation domains that occur in many proteins involved in membrane dynamics in a cell. The BAR domain is banana-shaped and binds to membrane via its concave face. It is capable of sensing membrane curvature by binding preferentially to curved membranes. BAR domains are named after three proteins that they are found in: Bin, Amphiphysin and Rvs.
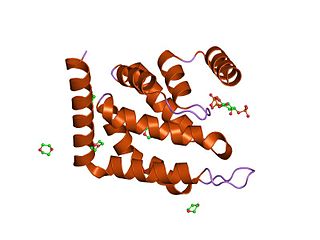
The epsin N-terminal homology (ENTH) domain is a structural domain that is found in proteins involved in endocytosis and cytoskeletal machinery.

Epsins are a family of highly conserved membrane proteins that are important in creating membrane curvature. Epsins contribute to membrane deformations like endocytosis, and block vesicle formation during mitosis.

Neural cell adhesion molecule L1-like protein also known as close homolog of L1 (CHL1) is a protein that in humans is encoded by the CHL1 gene.

Coatomer subunit beta is a protein that in humans is encoded by the COPB1 gene.

AP-2 complex subunit alpha-1 is a protein that in humans is encoded by the AP2A1 gene.

Clathrin heavy chain 1 is a protein that in humans is encoded by the CLTC gene.

Dynamin-1 is a protein that in humans is encoded by the DNM1 gene.
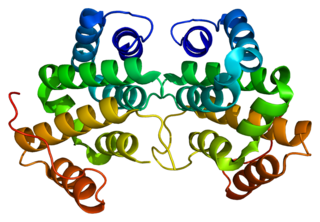
ADP-ribosylation factor-binding protein GGA2 is a protein that in humans is encoded by the GGA2 gene.

AP-1 complex subunit beta-1 is a protein that in humans is encoded by the AP1B1 gene.

Complexin-2 is a protein that in humans is encoded by the CPLX2 gene.

Synergin gamma also known as AP1 subunit gamma-binding protein 1 (AP1GBP1) is a protein that in humans is encoded by the SYNRG gene.

Epsin-1 is a protein that in humans is encoded by the EPN1 gene.

Epsin-2 is a protein that in humans is encoded by the EPN2 gene.

AP-2 complex subunit sigma is a protein that in humans is encoded by the AP2S1 gene.

Aftiphilin is a protein that in humans is encoded by the AFTPH gene. It forms a stable complex with p200 and synergin gamma. It contains a clathrin box with two known clathrin-binding sequence motifs, is involved in vesicle trafficking and is found in many eukaryotes.

U2AF homology motif (UHM) kinase 1, also known as UHMK1, is a protein which in humans is encoded by the UHMK1 gene.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.























