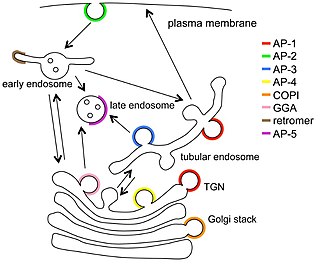
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.

Insulin-like growth factor 2 receptor (IGF2R), also called the cation-independent mannose-6-phosphate receptor (CI-MPR) is a protein that in humans is encoded by the IGF2R gene. IGF2R is a multifunctional protein receptor that binds insulin-like growth factor 2 (IGF2) at the cell surface and mannose-6-phosphate (M6P)-tagged proteins in the trans-Golgi network.

ADP-ribosylation factor 1 is a protein that in humans is encoded by the ARF1 gene.

ADP-ribosylation factor-binding protein GGA1 is a protein that in humans is encoded by the GGA1 gene.

AP-2 complex subunit mu is a protein that in humans is encoded by the AP2M1 gene.

AP-1 complex subunit mu-1 is a protein that in humans is encoded by the AP1M1 gene.

ADP-ribosylation factor-binding protein GGA3 is a protein that in humans is encoded by the GGA3 gene.

AP-1 complex subunit gamma-1 is a protein that in humans is encoded by the AP1G1 gene.

Sortilin (SORT1) is a protein that in humans is encoded by the SORT1 gene on chromosome 1. This protein is a type I membrane glycoprotein in the vacuolar protein sorting 10 protein (Vps10p) family of sorting receptors. While it is ubiquitously expressed in many tissues, sortilin is most abundant in the central nervous system. At the cellular level, sortilin functions in protein transport between the Golgi apparatus, endosome, lysosome, and plasma membrane, leading to its involvement in multiple biological processes such as glucose and lipid metabolism as well as neural development and cell death. Moreover, the function and role of sortilin is now emerging in several major human diseases such as hypertension, atherosclerosis, coronary artery disease, Alzheimer’s disease, and cancer. The SORT1 gene also contains one of 27 loci associated with increased risk of coronary artery disease.

AP-1 complex subunit beta-1 is a protein that in humans is encoded by the AP1B1 gene.

ADP-ribosylation factor 3 is a protein that in humans is encoded by the ARF3 gene.

AP-3 complex subunit delta-1 is a protein that in humans is encoded by the AP3D1 gene.

AP-1 complex subunit sigma-1A is a protein that in humans is encoded by the AP1S1 gene.

AP-1 complex subunit gamma-like 2 is a protein that in humans is encoded by the AP1G2 gene.

Synergin gamma also known as AP1 subunit gamma-binding protein 1 (AP1GBP1) is a protein that in humans is encoded by the SYNRG gene.

Rab GTPase-binding effector protein 1 is an enzyme that in humans is encoded by the RABEP1 gene. It belongs to rabaptin protein family.

In the fields of biochemistry and cell biology, the cation-dependent mannose-6-phosphate receptor (CD-MPR) also known as the 46 kDa mannose 6-phosphate receptor is a protein that in humans is encoded by the M6PR gene.

Low density lipoprotein receptor-related protein 3 (LRP-3) is a protein that in humans is encoded by the LRP3 gene.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. These proteins are synthesized in the ribosomes, processed in the endoplasmic reticulum and transported from the Golgi apparatus to the trans-Golgi network, and from there via small carrier vesicles to their final destination compartment. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.

The C-terminal domain ofBeta2-adaptin is a protein domain is involved in cell trafficking by aiding import and export of substances in and out of the cell.
























