
Streptomyces is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.

Hitachimycin, also known as stubomycin, is a cyclic polypeptide produced by Streptomyces that acts as an antibiotic. It exhibits cytotoxic activity against mammalian cells, Gram-positive bacteria, yeast, and fungi, as well as hemolytic activity; this is mediated by changes at the cell membrane and subsequent lysis. Owing to its cytotoxic activity against mammalian cells and tumors, it was first proposed as an antitumor antibiotic.

In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond.
Streptoalloteichus is a genus of bacteria within the family Pseudonocardiaceae that contains two known species:
Bohemic acid is a mixture of chemical compounds which is obtained through fermentation by actinobacteria species in the genus Actinosporangium (Actinoplanaceae). The name honors the Puccini opera La Bohème and many individual components of the acid carry the names of characters from La Bohème. Most of those components are antitumor agents and anthracycline antibiotics active against Gram-positive bacteria.
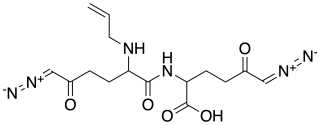
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica. It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.
The duocarmycins are members of a series of related natural products first isolated from Streptomyces bacteria in 1978. They are notable for their extreme cytotoxicity and thus represent a class of exceptionally potent antitumour antibiotics.
Streptomyces peucetius is a bacterium species in the genus Streptomyces.

Lavendamycin is a naturally occurring chemical compound discovered in fermentation broth of the soil bacterium Streptomyces lavendulae. Lavendamycin has antibiotic properties and anti-proliferative effects against several cancer cell lines. The use of lavendamycin as a cytotoxic agent in cancer therapy failed due to poor water solubility and non-specific cytotoxicity. The study of lavendamycin-based analogs designed to overcome these liabilities has been an area of research.

Kidamycin is an anthracycline antibiotic with anticancer activity. It was first synthesized from a strain of streptomyces bacteria isolated from a soil sample. In clinical trials, Kindamycin showed high effect against gram positive bacteria as well as multiple cancer models including Ehrlich ascites carcinoma, Sarcoma 180, NF-sarcoma, and Yoshida sarcoma.
Xanthomycin A is an antibiotic with in vitro antitumor activity isolated from Streptomyces.
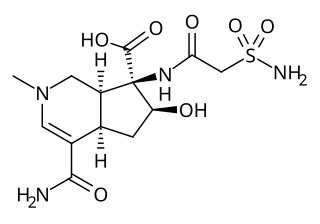
Altemicidin is monoterpene alkaloid first identified in isolates from marine actinomycetes in 1989. It may also be produced synthetically. Altemicidin displays both acaricidal and antitumor activity.
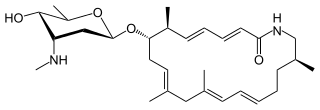
Vicenistatin is a macrolactam antibiotic synthesized by Streptomyces halstedii HC34. It was originally isolated from this bacterium in 1993. It includes the unusual starter unit methylaspartate.
Streptomyces verticillus is a species of Gram-positive bacteria in the genus Streptomyces. Whilst screening fermentation broths of this species for bioactivity in the early 1960s, Hamao Umezawa and colleagues at the Institute of Microbial Chemistry in Tokyo identified a family of glycopeptide antitumor antibiotics called the bleomycins. Examples of the bleomycins in clinical use include bleomycin A2 (also known as bleomycin) and bleomycin A5 (also known as pingyangmycin). Both are used to treat lymphomas (e.g. Hodgkin's lymphoma), head and neck cancer, and testicular cancer.
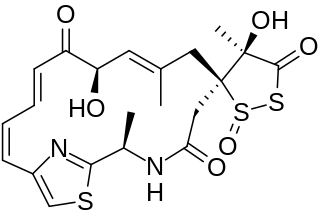
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria. The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.
Streptomyces capoamus is a bacterium species from the genus of Streptomyces which has been isolated from soil from Iceland. Streptomyces capoamus produces capomycin, ciclamycin O, ciclamycin 4, anthracycline, ciclacidin A, ciclacidin B and ciclamicin.
Streptomyces sanglieri is a bacterium species from the genus of Streptomyces which has been isolated from soil from a hay meadow. Streptomyces sanglieri produces the antibiotic lactonamycin Z.
Streptomyces tateyamensis is a bacterium species from the genus of Streptomyces which has been isolated from the sponge Haliclona from the pacific coastline of the city Tateyama in the Chiba prefecture in Japan. Streptomyces tateyamensis produces the antibiotic thiopeptin B.

C-1027 or Lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.

Tetracenomycin C is an antitumor anthracycline-like antibiotic produced by Streptomyces glaucescens GLA.0. The pale-yellow antibiotic is active against some gram-positive bacteria, especially against streptomycetes. Gram-negative bacteria and fungi are not inhibited. In considering the differences of biological activity and the functional groups of the molecule, tetracenomycin C is not a member of the tetracycline or anthracyclinone group of antibiotics. Tetracenomycin C is notable for its broad activity against actinomycetes. As in other anthracycline antibiotics, the framework is synthesized by a polyketide synthase and subsequently modified by other enzymes.











