
Carol A. Carter is an American microbiologist. She is Distinguished Professor in the Department of Microbiology and Immunology of the Renaissance School of Medicine at Stony Brook University, and member of the National Academy of Sciences. [1]

Carol A. Carter is an American microbiologist. She is Distinguished Professor in the Department of Microbiology and Immunology of the Renaissance School of Medicine at Stony Brook University, and member of the National Academy of Sciences. [1]
Carter grew up in Harlem with parents who stressed the importance of education, though they had only a seventh grade education. [2] Carter's interest in becoming a scientist was encouraged by a grade school teacher who gave her a book about inventors. [3] She earned her undergraduate degree from City College of New York [2] and went on to earn a Ph.D. from Yale University. [1]
Carter began graduate school in a bacteriophage laboratory before switching to animal virology, [3] training under virologist and epidemiologist Francis L. Black. [2] She discovered that measles virus has a nuclear phase of replication and that different strains of the virus cause acute measles versus subacute sclerosing panencephalitis. [2] She was influenced by Matthew Scharff of Albert Einstein College of Medicine, who pioneered the use of HeLa cells for cultivating animal viruses. [3] Carter did her postdoctoral work on double-stranded RNA viruses known as reoviruses in the laboratory of Aaron Shatkin. From her graduate work, Carter brought with her a trick for growing measles virus in culture: adding Kaopectate. [2]
In 1975, Carter joined the faculty of SUNY Stony Brook as assistant professor under department chair Joseph Kates, who discovered polyA on mRNA. [2] Carter continued working on reoviruses. After a sabbatical year studying the tumor virus SV40 in the laboratory of Carol Prives at Columbia University, she felt that the SV40 field was overcrowded, and sought a new field just at the time that HIV research was in its infancy. [3]
With poliovirus expert Eckard Wimmer, Carter studied how HIV cleaves a precursor polyprotein to make infectious particles, [4] [5] a strategy also used by poliovirus. [2] [6]
Carter investigated the mechanisms of HIV capsid particle assembly. Mentoring biochemist Lorna Ehrlich, the two helped demonstrate that recombinant HIV-1 p24 capsid protein could oligomerize in vitro. [3] [7] Together with X-ray crystallographer Michael Rossmann, and NMR spectroscopists Mike Summers and Wes Sundquist, Carter solved the structure of the p24 capsid protein. [8] [3]
Using a yeast-two hybrid library screen, Carter's graduate student Beth Agresta identified Tsg (tumor susceptibility gene)101, a novel cellular protein that interacts with HIV-1 Gag protein. [3] [9] Carter and her trainees Fadila Bouamr, Traci LaGrassa, Lynn VerPlank, Jay Goff and Gisselle Medina established how Tsg101-Gag interactions lead HIV to escape degradation, [10] undergo budding, [11] [12] [13] and effect particle assembly [14] [15] and release. [16] [17] [18] [19] Carter identified how Tsg101 recruits calcium signaling machinery [20] [21] to endosomal sorting complexes required for transport (ESCRT), [22] thus stabilizing viral assembly at the budding site. She continued working, with Jon Leis, to identify small molecules that target Tsg101 and other budding factors. [23] [24] [25]
Carter has received several awards: [1]
Carter enjoys walking on the beach and hosting gatherings for her husband, son and extended family. [3]

Feline immunodeficiency virus (FIV) is a Lentivirus that affects cats worldwide, with 2.5% to 4.4% of felines being infected.
The genome and proteins of HIV (human immunodeficiency virus) have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.

Viral infectivity factor, or Vif, is an accessory protein found in HIV and other lentiviruses. Its role is to disrupt the antiviral activity of the human enzyme APOBEC by targeting it for ubiquitination and cellular degradation. APOBEC is a cytidine deaminase enzyme that mutates viral nucleic acids.

Astroviruses (Astroviridae) are a type of virus that was first discovered in 1975 using electron microscopes following an outbreak of diarrhea in humans. In addition to humans, astroviruses have now been isolated from numerous mammalian animal species and from avian species such as ducks, chickens, and turkey poults. Astroviruses are 28–35 nm diameter, icosahedral viruses that have a characteristic five- or six-pointed star-like surface structure when viewed by electron microscopy. Along with the Picornaviridae and the Caliciviridae, the Astroviridae comprise a third family of nonenveloped viruses whose genome is composed of plus-sense, single-stranded RNA. Astrovirus has a non-segmented, single stranded, positive sense RNA genome within a non-enveloped icosahedral capsid. Human astroviruses have been shown in numerous studies to be an important cause of gastroenteritis in young children worldwide. In animals, Astroviruses also cause infection of the gastrointestinal tract but may also result in encephalitis, hepatitis (avian) and nephritis (avian).

Viral entry is the earliest stage of infection in the viral life cycle, as the virus comes into contact with the host cell and introduces viral material into the cell. The major steps involved in viral entry are shown below. Despite the variation among viruses, there are several shared generalities concerning viral entry.
Group-specific antigen, or gag, is the polyprotein that contains the core structural proteins of an Ortervirus. It was named as such because scientists used to believe it was antigenic. Now it is known that it makes up the inner shell, not the envelope exposed outside. It makes up all the structural units of viral conformation and provides supportive framework for mature virion.

The retroviral psi packaging element, also known as the Ψ RNA packaging signal, is a cis-acting RNA element identified in the genomes of the retroviruses Human immunodeficiency virus (HIV) and Simian immunodeficiency virus (SIV). It is involved in regulating the essential process of packaging the retroviral RNA genome into the viral capsid during replication. The final virion contains a dimer of two identical unspliced copies of the viral genome.

Tumor susceptibility gene 101, also known as TSG101, is a human gene that encodes for a cellular protein of the same name.
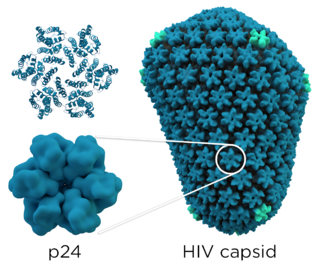
The p24 capsid protein is the most abundant HIV protein with each virus containing approximately 1,500 to 3,000 p24 molecules. It is the major structural protein within the capsid, and it is involved in maintaining the structural integrity of the virus and facilitating various stages of the viral life cycle, including viral entry into host cells and the release of new virus particles. Detection of p24 protein's antigen can be used to identify the presence of HIV in a person's blood and diagnose HIV/AIDS, however, more modern tests have taken their place. After approximately 50 days of infection, the p24 antigen is often cleared from the bloodstream entirely.
2F5 is a broadly neutralizing human monoclonal antibody (mAb) that has been shown to bind to and neutralize HIV-1 in vitro, making it a potential candidate for use in vaccine synthesis. 2F5 recognizes an epitope in the membrane-proximal external region (MPER) of HIV-1 gp41. 2F5 then binds to this epitope and its constant region interacts with the viral lipid membrane, which neutralizes the virus.
Retroviral matrix proteins are components of envelope-associated capsids of retroviruses. These proteins line the inner surface of viral envelopes and are associated with viral membranes.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.
Vpx is a virion-associated protein encoded by human immunodeficiency virus type 2 HIV-2 and most simian immunodeficiency virus (SIV) strains, but that is absent from HIV-1. It is similar in structure to the protein Vpr that is carried by SIV and HIV-2 as well as HIV-1. Vpx is one of five accessory proteins carried by lentiviruses that enhances viral replication by inhibiting host antiviral factors.

Susan Zolla-Pazner is an American research scientist who is a Professor of Medicine in the Division of Infectious Diseases and the Department of Microbiology at Mount Sinai School of Medicine and a guest investigator in the Laboratory of Molecular Immunology at The Rockefeller University, both in New York City. Zolla-Pazner's work has focused on how the immune system responds to the human immunodeficiency virus (HIV) and, in particular, how antibodies against the viral envelope develop in the course of infection.
Bovine foamy virus (BFV) is a ss(+)RNA retrovirus that belongs to the genus spumaviridae. Spumaviruses differ from the other six members of family retroviridae, both structurally and in pathogenic nature. Spumaviruses derive their name from spuma the latin for "foam". The 'foam' aspect of 'foamy virus' comes from syncytium formation and the rapid vacuolization of infected cells, creating a 'foamy' appearance.
Semotivirus is the only genus of viruses in the family Belpaoviridae. Species exist as retrotransposons in a eukaryotic host's genome. BEL/pao transposons are only found in animals. Semotivirus is the only genus currently recognized, the genus description corresponds to the family, Belpaoviridae description.
Paul Darren Bieniasz is a British-American virologist whose main area of research is HIV/AIDS. He is currently a professor of retrovirology at the Rockefeller University. He received the 2015 KT Jeang Retrovirology Prize and the 2010 Eli Lilly and Company Research Award. Bieniasz has been a Howard Hughes Medical Institute investigator since 2008.
Patricia Gail Spear is an American virologist. She is a professor emeritus of microbiology and immunology at Northwestern University in Evanston, Illinois. She is best known for her pioneering work studying the herpes simplex virus. Spear is a past president of the American Society for Virology and an elected member of the National Academy of Sciences.

Wesley I. Sundquist is an American biochemist. Sundquist is Samuels Chair, Distinguished Professor, and Chair of the University of Utah Department of Biochemistry. Sundquist's research focuses on cellular, molecular and structural biology of retroviruses, particularly Human Immunodefiency Virus (HIV), and on cellular membrane remodeling by the Endosomal Sorting Complexes Required for Transport (ESCRT) pathway.

Fadila Bouamr is a French-American virologist researching the molecular mechanisms that govern the assembly and egress of an infectious HIV-1 and the proteins involved in these processes. She is chief of the viral budding unit at the National Institute of Allergy and Infectious Diseases.