| |
| Names | |
|---|---|
| Preferred IUPAC name (3R,3aS,8aR)-6,8a-Dimethyl-3-(propan-2-yl)-2,3,4,5,8,8a-hexahydroazulen-3a(1H)-ol | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.366 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
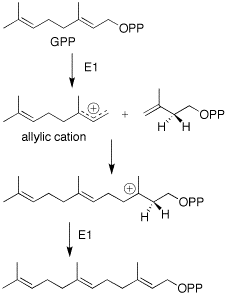
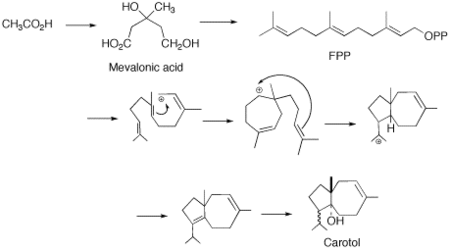
Carotol was first isolated by scientists Asahina and Tsukamoto in 1925. [1] It is one of the primary components found in carrot seed oil comprising approximately 40% of this essential oil. [2] This sesquiterpene alcohol is thought to be formed in carrot seeds (Daucus carota L., Umbelliferae) during the vegetation period. Additionally, studies have shown that carotol may be involved in allelopathic interactions expressing activity as an antifungal, herbicidal and insecticidal agent. [3]