 | |
| Names | |
|---|---|
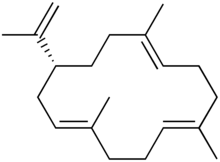
| Preferred IUPAC name (1E,5E,9E,12R)-1,5,9-Trimethyl-12-(prop-1-en-2-yl)cyclotetradeca-1,5,9-triene | |
| Other names (R,1E,5E,9E)-1,5,9-Trimethyl-12-(prop-1-en-2-yl)cyclotetradeca-1,5,9-triene Neocembrene-A | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C20H32 | |
| Molar mass | 272.47 g/mol |
| Boiling point | 150 to 152 °C (302 to 306 °F; 423 to 425 K) at 0.8 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Cembrene A, or sometimes neocembrene, is a natural monocyclic diterpene isolated from corals of the genus Nephthea . [1] It is a colorless oil with a faint wax-like odor.
Cembrene A is a trail pheromone for termites; [2] however, the chemical structure of cembrene is central to a very wide variety of other natural products found both in plants and in animals. [3] Pinus leucodermis tree bark and wood essential oils contain a high percentage of cembrene. [4]
Cembrenes are biosynthesized by macrocyclization of geranylgeranyl pyrophosphate. [3]