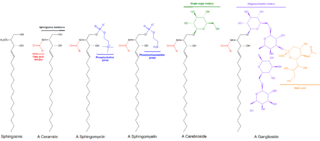
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, which are a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sphinx because of their enigmatic nature. These compounds play important roles in signal transduction and cell recognition. Sphingolipidoses, or disorders of sphingolipid metabolism, have particular impact on neural tissue. A sphingolipid with a terminal hydroxyl group is a ceramide. Other common groups bonded to the terminal oxygen atom include phosphocholine, yielding a sphingomyelin, and various sugar monomers or dimers, yielding cerebrosides and globosides, respectively. Cerebrosides and globosides are collectively known as glycosphingolipids.

Sphingomyelin is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a phosphoethanolamine head group; therefore, sphingomyelins can also be classified as sphingophospholipids. In humans, SPH represents ~85% of all sphingolipids, and typically make up 10–20 mol % of plasma membrane lipids.

Fumonisin B1 is the most prevalent member of a family of toxins, known as fumonisins, produced by multiple species of Fusarium molds, such as Fusarium verticillioides, which occur mainly in maize (corn), wheat and other cereals. Fumonisin B1 contamination of maize has been reported worldwide at mg/kg levels. Human exposure occurs at levels of micrograms to milligrams per day and is greatest in regions where maize products are the dietary staple.

Ceramides are a family of waxy lipid molecules. A ceramide is composed of sphingosine and a fatty acid joined by an amide bond. Ceramides are found in high concentrations within the cell membrane of eukaryotic cells, since they are component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer. Contrary to previous assumptions that ceramides and other sphingolipids found in cell membrane were purely supporting structural elements, ceramide can participate in a variety of cellular signaling: examples include regulating differentiation, proliferation, and programmed cell death (PCD) of cells.

Lipid signaling, broadly defined, refers to any biological cell signaling event involving a lipid messenger that binds a protein target, such as a receptor, kinase or phosphatase, which in turn mediate the effects of these lipids on specific cellular responses. Lipid signaling is thought to be qualitatively different from other classical signaling paradigms because lipids can freely diffuse through membranes. One consequence of this is that lipid messengers cannot be stored in vesicles prior to release and so are often biosynthesized "on demand" at their intended site of action. As such, many lipid signaling molecules cannot circulate freely in solution but, rather, exist bound to special carrier proteins in serum.

Sphingosine-1-phosphate receptor 2, also known as S1PR2 or S1P2, is a human gene which encodes a G protein-coupled receptor which binds the lipid signaling molecule sphingosine 1-phosphate (S1P).
In enzymology, sphingosine N-acyltransferases (ceramide synthases (CerS), EC 2.3.1.24) are enzymes that catalyze the chemical reaction of synthesis of ceramide:
In enzymology, a ceramide kinase, also abbreviated as CERK, is an enzyme that catalyzes the chemical reaction:

Alpha-N-acetylneuraminide alpha-2,8-sialyltransferase is an enzyme that in humans is encoded by the ST8SIA1 gene.

Ceramide glucosyltransferase is an enzyme that in humans is encoded by the UGCG gene.

Serine palmitoyltransferase, long chain base subunit 2, also known as SPTLC2, is a protein which in humans is encoded by the SPTLC2 gene. SPTLC2 belongs to the class-II pyridoxal-phosphate-dependent aminotransferase family.

BCL2-like 13 , also known as BCL2L13 or Bcl-rambo, is a protein which in humans is encoded by the BCL2L13 gene on chromosome 22. This gene encodes a mitochondrially-localized protein which is classified under the Bcl-2 protein family. Overexpression of the encoded protein results in apoptosis. As a result, it has been implicated in cancers such as childhood acute lymphoblastic leukemia (ALL) and glioblastoma multiforme (GBM). Alternatively spliced transcript variants have been observed for this gene, such as Bcl-rambo beta.

2-hydroxyacylsphingosine 1-beta-galactosyltransferase is an enzyme that in humans is encoded by the UGT8 gene.

Phosphatidylcholine:ceramide cholinephosphotransferase 1 is an enzyme that in humans is encoded by the SGMS1 gene.

Sphingosine-1-phosphate lyase 1 is an enzyme that in humans is encoded by the SGPL1 gene.

Delta-aminolevulinate synthase 1 also known as ALAS1 is a protein that in humans is encoded by the ALAS1 gene. ALAS1 is an aminolevulinic acid synthase.

Ceramide synthase 1 also known as LAG1 longevity assurance homolog 1 is an enzyme that in humans is encoded by the CERS1 gene.

Ceramide synthase 3 (CersS3), also known as longevity assurance homologue 3, is an enzyme that is encoded in humans by the CERS3 gene.
Ceramide synthase 4 (CerS4) is an enzyme that in humans is encoded by the CERS4 gene and is one of the least studied of the ceramide synthases.
Ceramide synthase 5 (CerS5) is the enzyme encoded in humans by the CERS5 gene.


















