
Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found and called actinium. Actinium gave the name to the actinide series, a group of 15 similar elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.
The actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinoid series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinoid chemistry to refer to any actinoid.

Francium is a chemical element with the symbol Fr and atomic number 87. Prior to its discovery, it was referred to as eka-caesium. It is extremely radioactive; its most stable isotope, francium-223, has a half-life of only 22 minutes. It is the second-most electropositive element, behind only caesium, and is the second rarest naturally occurring element. The isotopes of francium decay quickly into astatine, radium, and radon. The electronic structure of a francium atom is [Rn] 7s1, and so the element is classed as an alkali metal.

In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope is reached.
A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The seventh period contains 32 elements, tied for the most with period 6, beginning with francium and ending with oganesson, the heaviest element currently discovered. As a rule, period 7 elements fill their 7s shells first, then their 5f, 6d, and 7p shells in that order, but there are exceptions, such as uranium.

Marguerite Catherine Perey was a French physicist and a student of Marie Curie. In 1939, Perey discovered the element francium by purifying samples of lanthanum that contained actinium. In 1962, she was the first woman to be elected to the French Académie des Sciences, an honor denied to her mentor Curie. Perey died of cancer in 1975.
Actinium (89Ac) has no stable isotopes and no characteristic terrestrial isotopic composition, thus a standard atomic weight cannot be given. There are 32 known isotopes, from 205Ac to 236Ac, and 7 isomers. Three isotopes are found in nature, 225Ac, 227Ac and 228Ac, as intermediate decay products of, respectively, 237Np, 235U, and 232Th. 228Ac and 225Ac are extremely rare, so almost all natural actinium is 227Ac.
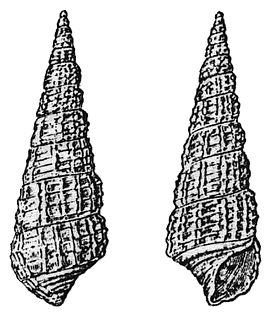
Cerithiidae, common name the cerithiids or ceriths, is a large family of medium-sized marine gastropods in the clade Sorbeoconcha.

Bittium is a genus of very small sea snails, marine gastropod molluscs in the family Cerithiidae, the horn snails.
Cerithidium australiense is a species of sea snail, a marine gastropod mollusk in the family Cerithiidae.
Cerithidium cerithinum is a species of sea snail, a marine gastropod mollusk in the family Cerithiidae.
Cerithidium diplax is a species of sea snail, a marine gastropod mollusk in the family Cerithiidae.
Cerithidium liratum is a species of sea snail, a marine gastropod mollusk in the family Cerithiidae.
Cerithidium perparvulum is a species of sea snail, a marine gastropod mollusk in the family Cerithiidae.

Cerithidium is a genus of sea snails, marine gastropod mollusks in the family Cerithiidae.
Actinium(III) oxide is a chemical compound containing the rare radioactive element actinium. It has the formula Ac2O3. It is similar to its corresponding lanthanum compound, lanthanum(III) oxide, and contains actinium in the oxidation state +3. Actinium oxide is not to be confused with Ac2O (acetic anhydride), where Ac is an abbreviation for acetyl instead of the symbol of the element actinium.

Actinium fluoride (AcF3) is an inorganic compound of actinium and fluorine.
Actinium(III) chloride is a chemical compound containing the rare radioactive element actinium. This salt has the formula AcCl3. Molecular weight of the compound is 333.378 g/mol.
Actinium(III) bromide is a radioactive white crystalline solid that is a salt of actinium. It is prepared by reacting actinium(III) oxide with aluminium bromide.
Actinium(III) iodide is the a chemical compound of the radioactive salt actinium. It is a white crystalline solid. This compound was made by heating actinium oxide with a mixture of aluminum metal and iodine at 700°C for two hours.







