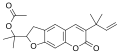
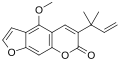
Salvinorin A is the main active psychotropic molecule in Salvia divinorum. Salvinorin A is considered a dissociative hallucinogen.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Galega officinalis, commonly known as galega or goat's-rue, is an herbaceous plant in the subfamily Faboideae of the legume family Fabaceae. It is native to parts of northern Africa, western Asia and Europe, but is widely cultivated and naturalised elsewhere. The plant has been extensively cultivated as a forage crop, an ornamental, a bee plant, and as green manure.

Ruta is a genus of strongly scented evergreen subshrubs, 20–60 cm tall, in the family Rutaceae, native to the Mediterranean region, Macaronesia and southwest Asia. About ten species are accepted in the genus. The most well-known species is Ruta graveolens.

Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin (PST). Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic shellfish poisoning (PSP).

Ruta graveolens, commonly known as rue, common rue or herb-of-grace, is a species of Ruta grown as an ornamental plant and herb. It is native to the Balkan Peninsula. It is grown throughout the world in gardens, especially for its bluish leaves, and sometimes for its tolerance of hot and dry soil conditions. It is also cultivated as a culinary herb, and to a lesser extent as an insect repellent and incense.

Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal. It is classified as a monoterpene.
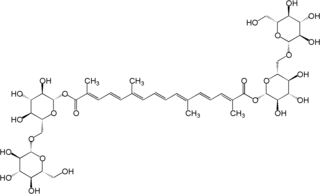
Crocin is a carotenoid chemical compound that is found in the flowers of crocus and gardenia. Its oxygen content also chemically makes it a xanthene. Crocin is the chemical primarily responsible for the color of saffron.

Zearalenone (ZEN), also known as RAL and F-2 mycotoxin, is a potent estrogenic metabolite produced by some Fusarium and Gibberella species. Specifically, the Gibberella zeae, the fungal species where zearalenone was initially detected, in its asexual/anamorph stage is known as Fusarium graminearum. Several Fusarium species produce toxic substances of considerable concern to livestock and poultry producers, namely deoxynivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS) and zearalenone. Particularly, ZEN is produced by Fusarium graminearum, Fusarium culmorum, Fusarium cerealis, Fusarium equiseti, Fusarium verticillioides, and Fusarium incarnatum. Zearalenone is the primary toxin that binds to estrogen receptors, causing infertility, abortion or other breeding problems, especially in swine. Often, ZEN is detected together with deoxynivalenol in contaminated samples and its toxicity needs to be considered in combination with the presence of other toxins.
Phytochemical Analysis is a bimonthly peer-reviewed scientific journal established in 1991 and published by John Wiley & Sons. It covers research on the utilization of analytical methodology in Plant Chemistry. The current editor-in-chief is Prof Satyajit Sarker and Managing Editor is Dr Lutfun Nahar.

Vulpinic acid is a natural product first found in and important in the symbiosis underlying the biology of lichens. It is a simple methyl ester derivative of its parent compound, pulvinic acid, and a close relative of pulvinone, both of which derive from aromatic amino acids such as phenylalanine via secondary metabolism. The roles of vulpinic acid are not fully established, but may include properties that make it an antifeedant for herbivores. The compound is relatively toxic to mammals.
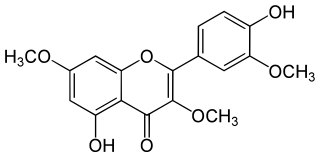
Pachypodol is a chemical compound classified as an O-methylated flavonol. It can be isolated from a variety of plants including Calycopteris floribunda, Pogostemon cablin, and Croton ciliatoglanduliferus.

Solamargine is a cytotoxic chemical compound that occurs in plants of the family Solanaceae, such as potatoes, tomatoes, and eggplants. It has been also isolated from Solanum nigrum fungal endophyte Aspergillus flavus. It is a glycoalkaloid derived from the steroidal alkaloid solasodine.

Genkwanin is an O-methylated flavone, a type of flavonoid. It can be found in the seeds of Alnus glutinosa, and the leaves of the ferns Notholaena bryopoda and Asplenium normale and Aquilaria.

Ruta chalepensis is a species of flowering plant in the Rutaceae family known by the common name fringed rue. It is native to Eurasia and North Africa. It has been found elsewhere as an introduced species. It is a perennial herb growing up to 80 centimeters tall. The leaves are compound, each divided into several segments which are subdivided into smaller leaflets. The inflorescence is a cluster of flowers, each with four or five bright yellow petals with rolled, fringed edges. The fruit is a textured capsule which is divided into pointed lobes.

Marmesin (nodakenetin) is a chemical compound precursor in psoralen and linear furanocoumarins biosynthesis.

Homoisoflavonoids (3-benzylidenechroman-4-ones) are a type of phenolic compounds occurring naturally in plants.
Streptomyces djakartensis is a bacterium species from the genus of Streptomyces which has been isolated from soil from Djakarta on Java in Indonesia. Streptomyces djakartensis produces niddamycins and N-acetyltryptamine.
Multidrug resistance pumps also known Multidrug efflux pumps are a type of efflux pump and P-glycoprotein. MDR pumps in the cell membrane extrudes many foreign substances out of the cells and some pumps can have a broad specificity. MDR pumps exist in animals, fungi, and bacteria and likely evolved as a defense mechanism against harmful substances. There are seven families of MDRs and are grouped by homology, energy source, and overall structure.

Barbatic acid is an organic compound that is made by some lichens. It is in the structural class known as depsides. It is particularly common in the genera Usnea and Cladonia.