
For the parent molecule 9,10-anthraquinone, see anthraquinone

Xanthoparmelia is a genus of foliose lichens in the family Parmeliaceae. This genus of lichen is commonly found in the United States, South America, southern Africa, Europe, Australia, and New Zealand.

Cryptothecia rubrocincta is a species of lichen in the fungal family Arthoniaceae. The species is distributed in subtropical and tropical locations throughout the southeastern United States, as well as Central and South America, and has been collected infrequently in a few locales in Africa. The body of the lichen forms continuous, circular crust-like patches on dead wood, readily recognizable by the prominent red pigment. The older, central region is covered with red, spherical to cylindrical granules. Moving outwards from the center, zones of color may be distinguished, the first gray-green, the second white, and finally a bright red cottony rim. The red and green colors of this unmistakable woodland lichen give the appearance of a Christmas wreath, suggestive of its common North American name, the Christmas (wreath) lichen. The red pigment, called chiodectonic acid, is one of several chemicals the lichen produces to help tolerate inhospitable growing conditions.
A spot test in lichenology is a spot analysis used to help identify lichens. It is performed by placing a drop of a chemical reagent on different parts of the lichen and noting the colour change associated with application of the chemical. The tests are routinely encountered in dichotomous keys for lichen species, and they take advantage of the wide array of lichen products produced by lichens and their uniqueness among taxa. As such, spot tests reveal the presence or absence of chemicals in various parts of a lichen. They were first proposed as a method to help identify species by the Finnish lichenologist William Nylander in 1866.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.
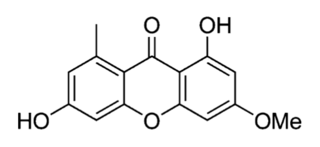
Griseoxanthone C is an organic compound in the structural class of chemicals known as xanthones. Its chemical formula is 1,6-dihydroxy-3-methoxy-8-methylxanthen-9-one, and its molecular formula is C15H12O5. It is found in a plant and some fungi, including a lichen.

Sekikaic acid is an organic compound in the structural class of chemicals known as depsides. It is found in some lichens. First isolated from Ramalina sekika, it is a fairly common lichen product in Ramalina and Cladonia, both genera of lichen-forming fungi. The species epithet of the powdery lichen Lepraria sekikaica refers to the presence of this substance—a rarity in genus Lepraria.

Barbatic acid is an organic compound that is made by some lichens. It is in the structural class known as depsides. It is particularly common in the genera Usnea and Cladonia.

Siegfried Huneck was a German chemist and lichenologist. Much of his scientific career was hampered by the political situation in the former German Democratic Republic. He rejected pursuing a career in academia, and instead ended up working at the Leibniz Institute of Plant Biochemistry, a public research institute, from 1969 until his retirement in 1993. Despite his relative isolation and restricted freedoms in East Germany, Huneck had numerous professional contacts both in Germany and abroad, and was a highly published scholar. Many of his more than 400 scientific publications dealt with the chemistry of lichen products. He was awarded the Acharius Medal for lifetime achievements in lichenology in 1996.
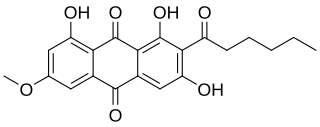
Solorinic acid is an anthraquinone pigment found in the leafy lichen Solorina crocea. It is responsible for the strong orange colour of the medulla and the underside of the thallus in that species. In its purified crystalline form, it exists as orange-red crystals with a melting point of 201 °C (394 °F).
Lecanora vinetorum is a rare species of crustose lichen in the family Lecanoraceae. Found in Central Europe, it was formally described as a new species in 1968 by lichenologists Josef Poelt and Siegfried Huneck. The type specimen was collected from the San Michele Appiano region of Trentino-Alto Adige ; there it was found growing on vineyard frames. The species epithet vinetorum refers to its habitat.

Parietinic acid is an organic compound in the structural class of chemicals known as anthraquinones. It is found in many species of the lichen family Teloschistaceae. The substance was first reported in the literature by the German chemist Walter Eschrich in 1958.
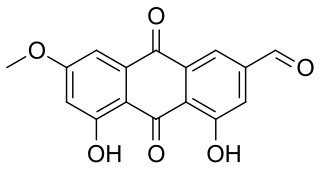
Fallacinal is an organic compound in the structural class of chemicals known as anthraquinones. It is found in many species of the lichen family Teloschistaceae.

Fallacinol (teloschistin) is an organic compound in the structural class of chemicals known as anthraquinones. It is found in some lichens, particularly in the family Teloschistaceae, as well as a couple of plants and non lichen-forming fungi. In 1936, Japanese chemists isolated a pigment they named fallacin from the lichen Oxneria fallax, which was later refined and assigned a tentative structural formula; by 1949, Indian chemists had isolated a substance from Teloschistes flavicans with an identical structural formula to fallacin. Later research further separated fallacin into two distinct pigments, fallacin-A and fallacin-B (fallacinol). The latter compound is also known as teloschistin due to its structural match with the substance isolated earlier.
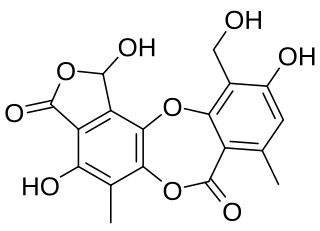
Connorstictic acid is an organic compound in the structural class of chemicals known as depsidones. It occurs as a secondary metabolite in many lichen species in several genera.

Confluentic acid is an organic compound belonging to the chemical class known as depsides. It serves as a secondary metabolite in certain lichens and plays a role in distinguishing closely related species within the genus Porpidia. In 1899, Friedrich Wilhelm Zopf isolated a compound from Lecidea confluens, which he initially named confluentin and noted for its melting point of 147–148 °C. This substance demonstrated the ability to turn litmus paper red and, when interacting with alkali, decomposed into carbon dioxide and phenol-like compounds. Zopf subsequently revised the chemical formula and melting point of the compound. Siegfried Huneck renamed it confluentinic acid in 1962, characterising it as optically inactive, with distinct colour reactions and solubility properties, and determined its molecular formula as C28H36O8.

Grayanic acid is an organic compound found in certain lichens, particularly Cladonia grayi, where it serves as a secondary metabolite with notable taxonomic importance. Identified in the 1930s, it is now recognised as a chemotaxonomic marker that helps distinguish closely related species within the Cladonia chlorophaea species group. Grayanic acid crystallises as colourless, needle-like structures, melts at approximately 186–189 °C (367–372 °F), and displays distinctive fluorescence under ultraviolet light, aiding in its detection and study.

Consalazinic acid is a chemical compound with the molecular formula C18H14O10. It is classified as a depsidone and is a secondary metabolite produced by a variety of lichens.

Chloroatranorin is a chemical compound with the molecular formula C19H17ClO8. It is a secondary metabolite produced by a variety of lichens and is a member of the depside class of compounds. It was first isolated from the oakmoss Evernia prunastri and characterized in 1934. It is the most common chlorine-containing depside in lichens, and has been identified in dozens of lichen species.

Protocetraric acid is a chemical compound with the molecular formula C18H14O9. It is a secondary metabolite produced by a variety of lichens and is classified as a depsidone.


















