
Cobalt(II) chloride is an inorganic compound of cobalt and chlorine, with the formula CoCl
2. It is a sky blue crystalline solid.

Copper(II) chloride is the chemical compound with the chemical formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate.

Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula [RhCl(PPh3)3] (Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. These metal salen complexes primarily find use as catalysts.
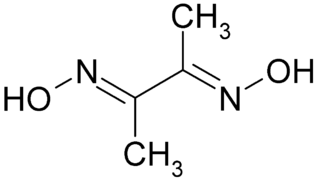
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts. Many related ligands can be prepared from other diketones, e.g. benzil.

Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.

Ferric acetate is the acetate salt of the coordination complex [Fe3O(OAc)6(H2O)3]+ (OAc− is CH3CO2−). Commonly the salt is known as "basic iron acetate". The formation of the red-brown complex was once used as a test for ferric ions.

Cyclooctadiene rhodium chloride dimer is the organorhodium compound with the formula Rh2Cl2(C8H12)2, commonly abbreviated [RhCl(COD)]2 or Rh2Cl2(COD)2. This yellow-orange, air-stable compound is a widely used precursor to homogeneous catalysts.
Organoiridium chemistry is the chemistry of organometallic compounds containing an iridium-carbon chemical bond. Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid. They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.
4-Toluenesulfonyl chloride (p-toluenesulfonyl chloride, toluene-p-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or TosCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.

Chloropentamminecobalt chloride is the dichloride salt of the coordination complex [Co(NH3)5Cl]2+. It is a red-violet, diamagnetic, water-soluble salt. The compound has been of academic and historical interest.

Pentamminechlororhodium dichloride is the dichloride salt of the coordination complex [RhCl(NH3)5]2+. It is a yellow, water-soluble solid. The salt is an intermediate in the purification of rhodium from its ores.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

Bis(triphenylphosphine)rhodium carbonyl chloride is the organorhodium complex with the formula [RhCl(CO)(PPh3)2]. This complex of rhodium(I) is a bright yellow, air-stable solid. It is the Rh analogue of Vaska's complex, the corresponding iridium complex. With regards to its structure, the complex is square planar with mutually trans triphenylphosphine (PPh3) ligands. The complex is a versatile homogeneous catalyst.

Dichlorotetrakis(pyridine)rhodium(III) chloride is the chloride salt of the coordination complex with the formula [RhCl2(pyridine)4]+. It is a yellow solid that crystallizes from water as the tetrahydrate, which converts to the monohydrate upon vacuum drying at 100 °C. It is prepared by treating rhodium trichloride with an excess of pyridine in the presence of a catalytic amount of a reductant.

Transition metal pyridine complexes encompas many coordination complexess that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.






















