The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling an organoboron species (R1-BY2) with a halide (R2-X) using a palladium catalyst and a base.

Nickel(II) chloride (or just nickel chloride), is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12; specifically [–(CH
2)
2–CH=CH–]2.

Platinum(II) chloride is the chemical compound PtCl2. It is an important precursor used in the preparation of other platinum compounds. It exists in two crystalline forms, but the main properties are somewhat similar: dark brown, insoluble in water, diamagnetic, and odorless.
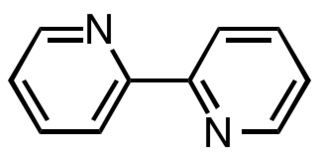
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications.
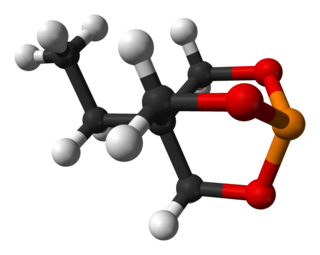
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a phosphite ester used as a ligand in organometallic chemistry. Trimethylolpropane phosphite is sometimes abbreviated to EtCage. It is a white solid that is soluble in organic solvents. It is also highly toxic.

Tripodal ligands are tri- and tetradentate ligands with C3 symmetry. They are popular in research in the areas of coordination chemistry and homogeneous catalysis. Because the ligands are polydentate, they do not readily dissociate from the metal centre.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

Rhodocene is a chemical compound with the formula [Rh(C
5H
5)
2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C
5H
5)
2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles. They are often called triazacyclohexanes or TACH's but this acronym is also applied to cis,cis-1,3,5-triaminocyclohexane
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.

1,1,1-Tris(diphenylphosphinomethyl)ethane, also called Triphos, is an organophosphorus compound with the formula CH3C[CH2PPh2]3. An air-sensitive white solid, it is a tripodal ligand ("three-legged") of idealized C3v symmetry. It was originally prepared by the reaction of sodium diphenylphosphide and CH3C(CH2Cl)3:
![Structure of [Ni(TACH)(H2O)3] (color code: blue = nitrogen, red = oxygen; dark blue = nickel). BAVNAN01.png](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ed/BAVNAN01.png/216px-BAVNAN01.png)













