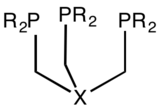
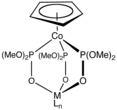
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is often the product-forming step in many catalytic processes. Since oxidative addition and reductive elimination are reverse reactions, the same mechanisms apply for both processes, and the product equilibrium depends on the thermodynamics of both directions.

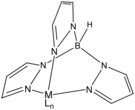
In coordination chemistry, a scorpionate ligand is a tridentate (three-donor-site) ligand that binds to a central atom in a fac manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then little-known DuPont chemist of Ukrainian descent, Swiatoslaw Trofimenko. Trofimenko called this discovery "a new and fertile field of remarkable scope".

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Tris(2-aminoethyl)amine is the organic compound with the formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Tris(2-aminoethyl)amine is commonly abbreviated as tren or TREN. It is used a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry.

In coordination chemistry, denticity refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate. Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
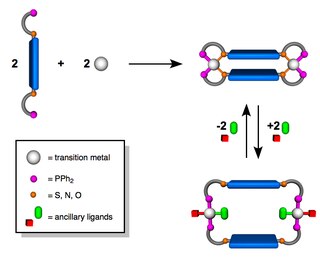
The Weak-Link Approach (WLA) is a supramolecular coordination-based assembly methodology, first introduced in 1998 by the Mirkin Group at Northwestern University. This method takes advantage of hemilabile ligands -ligands that contain both strong and weak binding moieties- that can coordinate to metal centers and quantitatively assemble into a single condensed ‘closed’ structure. Unlike other supramolecular assembly methods, the WLA allows for the synthesis of supramolecular complexes that can be modulated from rigid ‘closed’ structures to flexible ‘open’ structures through reversible binding of allosteric effectors at the structural metal centers. The approach is general and has been applied to a variety of metal centers and ligand designs including those with utility in catalysis and allosteric regulation.

Trisoxazolines are a class of tridentate, chiral ligands composed of three oxazoline rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths, due to the chelate effect. The ligands have been investigated for molecular recognition and their complexes are used in asymmetric catalysts and polymerisation.
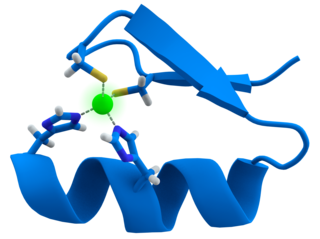
Transition metal thiolate complexes are metal complexes containing thiolate ligands. Thiolates are ligands that can be classified as soft Lewis bases. Therefore, thiolate ligands coordinate most strongly to metals that behave as soft Lewis acids as opposed to those that behave as hard Lewis acids. Most complexes contain other ligands in addition to thiolate, but many homoleptic complexes are known with only thiolate ligands. The amino acid cysteine has a thiol functional group, consequently many cofactors in proteins and enzymes feature cysteinate-metal cofactors.

Tris(oxazolinyl)borate compounds are a class of tridentate ligands; often abbreviated ToR, where R is the substituent on the oxazoline ring. Most commonly the substituent is either a methyl, propyl, tert-butyl or hydrogen. The formation of anionic boron backbone with addition of a phenyl group on boron allows the ligand to strongly bind to the metal center. It results in a more robust complex.

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles. They are often called triazacyclohexanes or TACH's but this acronym is also applied to cis,cis-1,3,5-triaminocyclohexane.

Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.

cis,cis-1,3,5-Triaminocyclohexane is an organic compound with the formula (CH2CHNH2)3. It is a triamine. Of the many isomers possible for triaminocyclohexane, the cis,cis-1,3,5-derivative has attracted attention because it is a common tripodal ligand, abbreviated as tach. It is a colorless oil. It is a popular tridentate ligand in coordination chemistry.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.

Transition metal complexes of pyridine-N-oxides encompass coordination complexes that contain pyridine-N-oxides as ligands. Particularly common are the octahedral homoleptic complexes of the type [M(ONC5H5)6]2+ where M = Mn(II), Fe(II), Co(II), Ni(II). Many variations of pyridine N-oxide are known, such as the dioxides of 2,2'- and 4,4'-2,2'-bipyridine. Complexes derived from the trioxide of terpyridine have been crystallized as well.

![Structure of [Ni(TACH)(H2O)3] (color code: blue = nitrogen, red = oxygen, dark blue = nickel). BAVNAN01.png](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ed/BAVNAN01.png/144px-BAVNAN01.png)